Neuroblastoma Research News Updates
1 - 8 of 8
Children’s Hospital of Philadelphia Announces Endowed Chair in Pediatric Neuroblastoma Research
Published on

CHOP recipient, physician-scientist Dr. Yael Mossé, is innovating targeted, less toxic therapies for cancer affecting mostly infants and young children.
CHOP Scientists Identify Gene Variants that Increase Neuroblastoma Risk
Published on
By identifying common gene variants that raise the risk of an aggressive form of the childhood cancer neuroblastoma, researchers may assist diagnostic efforts.
New Drug May Overcome Treatment Resistance in a High-risk Children’s Cancer
Published on
CHOP researchers have identified a powerful new drug with “unparalleled” strength against refractory types of neuroblastoma.
Change in a Single DNA Base Drives a Childhood Cancer
Published on
CHOP pediatric oncology researchers have pinpointed a crucial change in a single DNA “letter” that predisposes children to an aggressive form of neuroblastoma.
CHOP Is a Founding Member of New NIH-Funded Research Consortium to Test New Cancer Treatments for Children
Published on

Building on its decades-long investigations in the biology and treatment of neuroblastoma, CHOP will lead the consortium’s neuroblastoma research program.
Pinpointing Mutations in a Relapsed Cancer May Lead to Better Treatments
Published on

CHOP neuroblastoma researchers have detailed how cancer-driving mutations evolve during chemotherapy, and they aim to exploit this knowledge to design better treatments for children.
Nanoparticles May Exploit Tumor Weaknesses to Selectively Attack Cancers
Published on
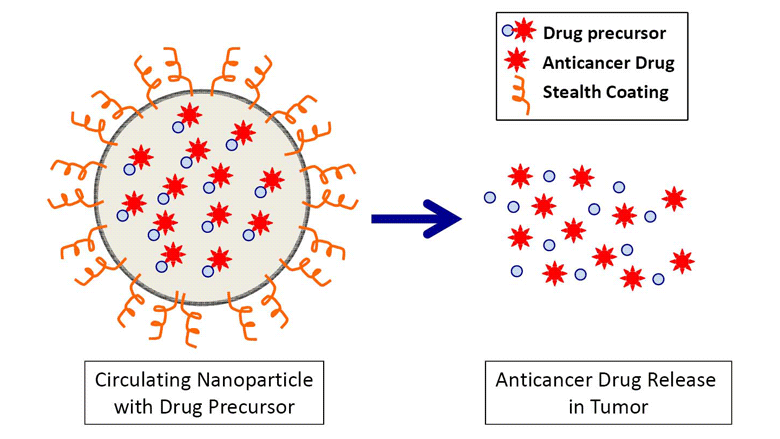
Delving into the world of the extremely small, CHOP researchers are exploring how nanoparticles can precisely deliver anticancer drugs to attack neuroblastoma.
Classification of Gene Mutations in a Children’s Cancer May Point to Improved Treatments
Published on
CHOP and Penn Medicine experts are refining their diagnostic tools to predict which patients are more likely to respond to drugs called ALK inhibitors that target such mutations, setting the stage for a phase 3 clinical trial.