Tiny Tools, Big Results
How cardiac catheterization is changing the way heart disease is diagnosed and treated in children
Published on
Published on
Each device is smaller than a dime but can perform a herculean task: replacing a failing pulmonary valve, plugging an abnormal connection in the heart, redirecting blood flow to the lungs or halting leaks in the lymphatic system.
A generation ago, there were limited options for children with serious cardiac or lymphatic conditions like these. For the lucky few, surgery was possible, but many others could not be saved.
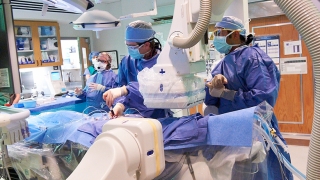 Today, thousands of children with congenital heart disease (CHD) and lymphatic disorders are not just alive — they're thriving — thanks to innovative cardiac catheterization procedures pioneered and perfected by world-leading cardiologists at Children's Hospital of Philadelphia (CHOP).
Today, thousands of children with congenital heart disease (CHD) and lymphatic disorders are not just alive — they're thriving — thanks to innovative cardiac catheterization procedures pioneered and perfected by world-leading cardiologists at Children's Hospital of Philadelphia (CHOP).
Decades of research, clinical experience and device advances have led to a major shift in the way heart disease is treated at CHOP — and around the world. No longer is surgery the only, or even best, option. Instead, many heart conditions are being treated with cardiac catheterization, often called "a cath," a noninvasive procedure where long, flexible tubes called catheters are threaded through an artery or vein in the leg, up through the blood vessels and into the heart.
Since the 1980s, the Cardiac Center at CHOP has used catheters to map the structure and function of children's hearts. This helps clinicians identify heart anomalies and plan treatments. As cardiac imaging has improved, so has the quality and quantity of information clinicians can gather from these procedures.
"We are closing holes in the heart, opening narrow blood vessels, replacing faulty valves and helping kids with complicated heart conditions delay or avoid open-heart surgery," says Jonathan J. Rome, MD, Associate Chief of Clinical Affairs and attending cardiologist at CHOP. About 1,500 cardiac catheterization procedures are performed each year at CHOP. The benefits for children include a decreased use of anesthesia, less risk of infection and a quicker recovery time.
In the past year, CHOP's Cardiac Center has performed more than 75,000 diagnostic tests on patients and nearly 900 cardiothoracic surgeries. The large volume of patients assessed and treated gives CHOP a unique perspective on all types of congenital heart disease (CHD).
Many children with CHD are successfully treated early in their lives with staged reconstructive heart surgery. Often though, these children develop related issues later that need to be addressed. Such was the case for Casey and Tayvin.
 Casey was 18 when his pulmonary valve began to leak. Like many children treated for tetralogy of Fallot (TOF), as a baby, Casey needed a valve replacement as he entered adulthood.
Casey was 18 when his pulmonary valve began to leak. Like many children treated for tetralogy of Fallot (TOF), as a baby, Casey needed a valve replacement as he entered adulthood.
Matthew Gillespie, MD, an attending cardiologist and Medical Director of the Cardiac Catheterization Laboratory with expertise in interventional cardiology, suggested a transcatheter valve replacement instead of heart surgery. The innovative procedure to deliver a new value to the heart via catheter is safer than surgery, but requires a precisely fit valve. Unfortunately, all the medically available valves at the time were not a good fit for Casey.
Because Casey's condition wasn't yet affecting his quality of his life and doctors knew that new devices were in development, doctors decided to wait for a suitable device to become available instead of automatically switching to a surgical solution. CHOP doctors performed a cardiac catheterization to take precise measurements of Casey's internal anatomy, then built a 3D model of Casey's heart.
After two years of monitoring, CHOP doctors believed they'd found the right solution for Casey: the HarmonyTM Transcatheter Pulmonary Valve (TPV), a finger-sized, self-expanding metal frame. The device was still in clinical trials, but Dr. Gillespie and his team were confident the valve was a good fit for Casey after analyzing it in conjunction with the 3D heart model of his heart.
In August 2018, Casey became the first human recipient of the Harmony TPV at CHOP. Today, Casey is 22 and enjoying an active adult life.
 Tayvin was born with and treated for hypoplastic left heart syndrome. When he was 4, his lymphatic system began leaking a large amount of fluid into his chest. He had developed chylothorax.
Tayvin was born with and treated for hypoplastic left heart syndrome. When he was 4, his lymphatic system began leaking a large amount of fluid into his chest. He had developed chylothorax.
Tayvin's doctors in California, where he lived, tried to halt the flow of fluid, but their treatments didn't work. They referred Tayvin's family to CHOP, where doctors had developed a new procedure to repair leaks in children's lymphatic systems.
The family met with Yoav Dori, MD, a pediatric cardiologist at CHOP, who directs the Jill and Mark Fishman Center for Lymphatic Disorders, and a few days later, Dr. Dori used dynamic contrast MR lymphangiography (CDMRL), an advanced imaging test, to confirm the location of Tayvin's leaks, then performed a thoracic duct embolization to stop the flow of lymph that was leaking into his lungs.
Tayvin showed a dramatic improvement soon after the procedure, and is now back home with his family enjoying his favorite activities.
While staged reconstructive heart surgery is the best option for many children with complex cardiac defects that affect the structure and function of the heart, sometimes, there's a heart condition so rare and so different, that even experts haven't encountered it.
 Such was the case with Amelia. A routine prenatal ultrasound revealed Amelia's heart was enlarged. After more tests and scans, Amelia's family learned she had a pulmonary artery venous fistula, a rare, abnormal connection between an artery and vein in the lungs causing blood to pass through the lungs without receiving enough oxygen. Before birth, Amelia was at risk of heart failure. After birth, she was at risk of oxygen-deprivation.
Such was the case with Amelia. A routine prenatal ultrasound revealed Amelia's heart was enlarged. After more tests and scans, Amelia's family learned she had a pulmonary artery venous fistula, a rare, abnormal connection between an artery and vein in the lungs causing blood to pass through the lungs without receiving enough oxygen. Before birth, Amelia was at risk of heart failure. After birth, she was at risk of oxygen-deprivation.
Doctors from the Cardiac Center and the Center for Fetal Diagnosis and Treatment collaborated to keep Amelia stable during her prenatal development. At 37-weeks gestation, CHOP experts prepared for an IMPACT delivery (Immediate Postpartum Access to Cardiac Therapy).
IMPACT was developed by the CHOP clinical team and specifically designed to manage heart conditions that required urgent cardiovascular care as soon as the baby is born via caesarean section. Within minutes of Amelia's birth in October 2018, Dr. Rome assessed her and performed an angiogram to locate and identify the fistula. Then, using a catheter, he inserted an Amplatzer™ vascular plug, a special expanding metal device to close the fistula.
Less than two hours after Amelia was born, her parents were told the good news: The tiny device successfully closed the fistula, her blood was properly circulating, and she would be fine. Today, Amelia is a healthy child with no restrictions.
While cardiologists have been using catheterization for more than a decade, the number of procedures for some heart defects — such as replacing a transcatheter pulmonary valve or closing a patent ductus arteriosus (PDA) — have more than doubled at CHOP In the past five years.
Both therapeutic procedures require catheters to transport specialty-made devices into the heart to be correctly placed by interventional cardiologists. This type of innovation is happening every day at CHOP as clinicians push to find solutions for every child with CHD.
"The depth and breadth of the experience and talent at CHOP just doesn't exist anywhere else. We are leaders in the device realm, partnering with manufacturers to pioneer new valves and devices," Dr. Gillespie says.
Physicians and researchers at CHOP are also actively involved in cardiac catheterization research. They've published extensively about new procedures, new devices and new options for existing devices. CHOP is often the first to implement and use cutting-edge new technologies in multisite and individual clinical studies.
"New technologies don't scare us; they inspire us to find the best solutions for our patients and families," he adds. Put simply: If a catheterization procedure or intervention is possible; it can be done at CHOP.
 Julia's treatment is a good example of the type of novel solutions that CHOP has become known for. Born with and treated for heterotaxy syndrome, Julia developed pulmonary arteriovenous malformations (AVMs), causing a progressive decrease in her blood oxygen level. She was exhausted and in pain all the time.
Julia's treatment is a good example of the type of novel solutions that CHOP has become known for. Born with and treated for heterotaxy syndrome, Julia developed pulmonary arteriovenous malformations (AVMs), causing a progressive decrease in her blood oxygen level. She was exhausted and in pain all the time.
By the time she was in eighth grade, her condition had deteriorated so much that doctors considered putting her on the list for a heart transplant. But her heart wasn't failing — her problem was that her blood wasn't picking up enough oxygen as it passed through her lungs.
One of the doctor's on Julia's team, Dr. Rome, believed there was a way to reroute the blood inside her body without surgery. The team made a 3-D model of her anatomy and performed numerous tests before developing a way to rechannel her blood flow using special stents delivered through catheters.
Unfortunately, the procedure had never been tried before and there was no guarantee it would work. But, Dr. Rome was confident the new procedure was a good alternative to a heart transplant and presented the idea to Julie and her parents. They agreed.
During the procedure, stents were inserted using catheterization through her blood vessels. Within two months, Julia's energy began to return. Now a young adult, Julia says CHOP changed her life.
"If it weren't for the new discoveries being made in the Cardiac Center, I wouldn't be able to live out my dream of being in nursing school," Julia says.
A new therapy is now available at Children's Hospital for patent ductus arteriosus (PDA) closure in extremely low birth weight (ELBW) infants. PDA is one of the most common congenital heart defects in premature babies. It occurs when the ductus arteriosus — a blood vessel in the fetal heart that is supposed to close after birth — remains open. As a result, babies have difficulty breathing normally due to the increased blood flow to the lungs. While small PDAs often close on their own, a large PDA can lead to life-threatening problems.
Until recently, only two devices approved by the U.S. Food and Drug Administration (FDA) existed to "plug" holes in the heart for pediatric patients. Neither were small enough to help extremely premature newborns.
Clinicians at CHOP, recognizing the need for a new way to help ELBW babies born with PDAs, participated in a clinical trial to perfect a tiny, self-expanding device the size of a pea to seal this troublesome heart defect. CHOP was the leading center in the clinical trial for the Amplatzer Piccolo Occluder. Armed with positive outcomes and research at CHOP, in January 2019, the FDA officially approved the Piccolo device as a treatment option for premature babies with PDA.
Four months later, the device was used to save Zylah's life. Born at less than 26 weeks gestation, Zylah weighed only 1 pound, 9 ounces. She experienced a host of issues related to her prematurity — including a PDA. The heart defect meant Zylah's body was recirculating already oxygenated blood to her lungs — flooding them with extra volume. After multiple blood transfusions at the suburban Pennsylvania hospital where she was born, Zylah was transferred to CHOP for advanced care and treatment.
CHOP cardiologists and interventionalists examined Zylah and determined the newly approved Piccolo device was her best chance for survival. On May 16, 2019, they performed the procedure to implant the device. Within days, Zylah's condition began to improve. But she still had a lot of growing to do to catch up developmentally to a full-term baby.
Today, 5-month-old Zylah is thriving. She's back home with her mom, is a healthy 9½ pounds, and no longer needs supplemental oxygen or IV nutrition.
"CHOP saved my baby's life," says Zylah's mom, Zakira. "She's doing great, and I can't wait to see what she'll do next."
These are just a few examples of children who have a brighter future because of cardiac catheterization. There are hundreds of others at CHOP and thousands around the world who have benefited from the dramatic increase in the use of cardiac catheterization. Not only has cardiac catheterization proven to be a successful tool for cardiologists, in some cases it's been more effective than more traditional open-heart surgery.
Cardiac catheterization cannot be used to treat all cardiac conditions. But, it helps clinicians get a closer look at heart anomalies. By mapping the heart, its structures and pathways, clinicians can better plan treatment — whether it's a catheterization procedure or not.
"Our patients and families trust us to find the best solutions for their situations, and we take that trust very seriously," Dr. Gillespie says. "That's why we strive so hard to find new devices to help patients, to retrofit existing devices, to track long-term outcomes and to compare our research with that of other facilities across the country. Our patients' lives depend on it."