Management of Myelomeningocele Study
The federally sponsored Management of Myelomeningocele Study (MOMS), co-led by experts at Children’s Hospital of Philadelphia, reported results of a clinical trial of fetal surgery for myelomeningocele, the most severe form of spina bifida.
The randomized, controlled clinical trial was initiated and funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The trial’s results directly compared outcomes of prenatal surgery versus postnatal repair in 183 patients.
The study showed that fetal surgery for spina bifida greatly reduces the need to divert fluid from the brain, improves mobility and improves the chances that a child will be able to walk independently.
Two and a half years after fetal surgery, children with spina bifida were better able to walk, when compared to children who received surgery shortly after birth. Patients who received fetal surgery also scored better on tests of motor function. Within a year after fetal surgery, they were less likely to need a shunt, a surgically implanted tube that drains fluid from the brain.
Specifically, the study found that prenatal repair resulted in:
- Reduced need for ventricular shunting (a procedure in which a thin tube is introduced into the brain’s ventricles to drain fluid and relieve hydrocephalus), as measured at 12 months of age
- Reduced incidence or severity of neurologic effects caused by the spine’s exposure to amniotic fluid, such as impaired motor and sensory function of the legs
- Improved ambulation, as measured at 30 months of age
- Reversal of the hindbrain herniation component of the Chiari II malformation
The trial demonstrated that outcomes after prenatal spina bifida treatment are improved to the degree that the benefits of the surgery outweigh the maternal risks. Study results were published in February 2011 in The New England Journal of Medicine.
For more information:
- Read “A randomized trial of prenatal versus postnatal repair of myelomeningocele”
- Read a press release highlighting the study's results
- See more basic and clinical spina bifida research findings
- See CHOP's fetal MMC repair outcomes following the MOMS trial
- Read about a study of the full cohort from the original MOMS trial that shows a clear-cut benefit in outcomes at age 30 months, including the ability to walk independently
Follow-up through 2 1/2 years of age for study participants was completed in October 2013.
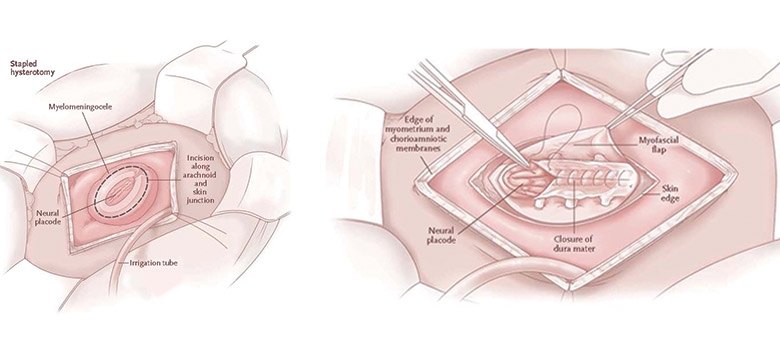 Prenatal repair of myelomeningocele. Source: N. Scott Adzick, MD et al., “A Randomized Trial of Prenatal versus Postnatal Repair of Myelomeningocele,” N Engl J Med. 2011 Mar 17;364(11):993-1004. Epub 2011 Feb 9.
Prenatal repair of myelomeningocele. Source: N. Scott Adzick, MD et al., “A Randomized Trial of Prenatal versus Postnatal Repair of Myelomeningocele,” N Engl J Med. 2011 Mar 17;364(11):993-1004. Epub 2011 Feb 9.
MOMS2
In 2020, researchers published new findings in follow-up study known as “MOMS2: Follow up of the Management Of Myelomeningocele Study.” These findings, published in Pediatrics, show significant physical and emotional benefits a decade later in school-age children who received corrective surgery in the womb for myelomeningocele.
Read more about the findings from phase two of the Management of Myelomeningocele Study.
MOMS3
Phase three of MOMS, known as “Follow-up in the Teen and Young Adult Years to the Management of Myelomeningocele Study: MOMS3” — is monitoring the health outcomes of the children and mothers who participated in the randomized trial. The purpose of the study is to compare the long-term effects of prenatal surgery and postnatal surgery for MMC with respect to the child’s adaptive behavior, physical and cognitive function, health and well-being, and the future reproductive health of the mother. Read more about the MOMS3 study.