Foundation news
Dream Job
A class of high school seniors gets the ultimate graduation present: guaranteed employment at CHOP.

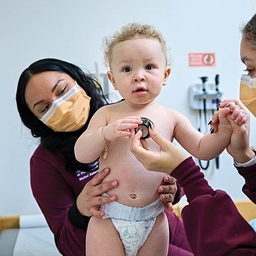
On a Mission
A visionary program at CHOP tackles one of the greatest challenges in pediatric cardiology.
Medicine — and More
Families in need that come to the ED get unexpected support, from diapers and food to a listening ear and compassionate concern.

A Day in the Life: Gino Poliziani, LSW
Follow Gino Poliziani, LSW, a social worker in the Cardiac Intensive Care Unit.

Embracing Uncertainty
Breakthrough research into the ethics of genetic testing has earned Katharine P. Callahan, MD, MSME, a new title: Wunderkind.

A Place of Hope and Healing
Using children’s strengths as a path to recovery.

Mastery Schools, Children’s Hospital of Philadelphia Announce Innovative Healthcare High School to Address Local Healthcare Workforce Shortages with Support from Bloomberg Philanthropies
Philadelphia joins Bloomberg Philanthropies’ first-of-its-kind initiative that connects students to job opportunities with family-sustaining wages in 10 communities across the U.S.

Annual Philly Spin-In Raises More than $1M Benefiting Children’s Hospital of Philadelphia’s Cardiac Center
On Saturday, March 9 and Sunday, March 10, more than 200 teams rode for little hearts at the eighth annual Philly Spin-In, raising funds and awareness for cardiac care and research.

Cheers for CHOP presented by BAYADA raises $650,000 for Children’s Fund
Children’s Hospital of Philadelphia (CHOP) hosted Cheers for CHOP presented by BAYADA at The Fillmore to benefit our Children’s Fund.
The Wyss Foundation provides $5 Million in funding for Children’s Hospital of Philadelphia’s Wyss/Campbell Center for Thoracic Insufficiency Syndrome
Children’s Hospital of Philadelphia (CHOP) is thrilled to announce a gift of $5 million from the Wyss Foundation to support innovations that improve the lives of patients with thoracic insufficiency syndrome (TIS).