If hepatitis B virus was a superhero, its secret power would be its stealth. In fact, hepatitis B is so stealthy that many people don’t even know they are infected until decades after their initial exposure when they are diagnosed with liver disease.
Hepatitis B is a virus that replicates in liver cells. While many people recover after their initial infection, some go on to develop chronic hepatitis B. Many never know they were infected — or that they are so-called “carriers” — until they are diagnosed with hepatitis (inflammation of the liver), cirrhosis (severe liver damage) or liver cancer.
Many people consider hepatitis B an infection of only high-risk individuals, such as sex workers and injection drug users, but that viewpoint discounts hepatitis B’s superpower. Let’s explore more about how this virus can hide in plain sight and why that has led to the current vaccine recommendations to protect against hepatitis B.
There’s more to the spread of hepatitis B than meets the eye
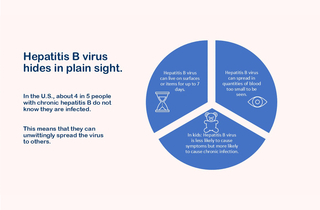
Most people focus on the spread of hepatitis B through sex and injection drug use, but anyone can get hepatitis B, including children. Before hepatitis B vaccine was recommended for all children, each year about 18,000 infants and children were infected with the virus. About half of those children were infected during delivery or shortly thereafter because their mothers were infected. The other 9,000 often never knew when, where or how they were exposed to the virus. Three features contribute to hepatitis B’s stealthiness:
Invisible blood
While hepatitis B replicates in the liver, the virus, as well as proteins from the virus, circulate in the blood of infected individuals. This is how it is spread from person to person. Some people infected with hepatitis B virus have about 10 million to 1 billion hepatitis B virus particles in every milliliter of blood (a milliliter is about one-fifth of a teaspoon). But even among those with lower quantities (e.g., 100 to 1,000 virus particles per milliliter of blood), hepatitis B can be transmitted in amounts of blood not visible to the naked eye. Some body fluids have also been demonstrated to spread the virus, likely because of undetectable quantities of blood, including saliva, semen and vaginal fluids.
The presence of the virus in small or invisible amounts of blood means transmission can occur in unexpected ways or at places that people might not think about. For example:
- Although hepatitis B virus DNA and hepatitis B virus surface protein can be found in breast milk from mothers who are hepatitis B positive, a baby won’t be infected by the milk. However, if that mother has cracked or bleeding skin or nipples, the baby can be exposed to the virus while breastfeeding. In fact, the recommendation is that in this situation a hepatitis B-positive mother should not breastfeed until healed because of this likelihood.
- Hepatitis B can also be spread by other household contacts as well as people in day cares or schools and other non-household contacts. Examples include contact with blood from cuts in skin; pre-chewing of food; sharing gum or other foods; sharing toothbrushes, razors, utensils or other items that might contain undetectable amounts of blood, like washcloths or clothing; and contact with blood during sports or other physical activities.
Survival time
Hepatitis B virus is considered a hardy, or robust, virus, meaning it doesn’t die quickly once outside of the body. In fact, it can live for up to seven days on surfaces or objects that have been touched by an infected person’s blood or infected body fluids, including clothing, utensils, washcloths or bedding. People have also been infected in medical, dental, long-term care, and other facilities. These cases can occur from improperly sterilized medical equipment, needle sticks, or other exposures. For example, cases have resulted from acupuncture as well as tattooing and assisting with blood glucose monitoring.
Characteristics of infection
Two aspects of hepatitis B infections also contribute to the ease with which this virus can hide among us. First, only about 3 to 5 of every 10 older children and adults experience symptoms when they are initially exposed to the virus. In children less than 5 years of age, only 1 in 10 experience symptoms. As a result, it is easy for a person to be infected and not know. Second, the younger a person is when they are exposed, the more likely that they will become chronically infected. Fewer than 5 of every 100 healthy adults will become chronically infected, but about 3 of 10 children infected between 1 and 4 years of age and about 9 of 10 children infected during the first year of life will become chronically infected. Together these characteristics position the youngest children to unwittingly become chronically infected carriers. About 1 of every 4 people infected at birth or during early childhood will die earlier than others of the same age because of liver disease caused by hepatitis B.
The bottom line
In the U.S., about 4 in 5 people with chronic hepatitis B infections do not know they have this infection. They don’t have symptoms, and if they were exposed as children, they may be carriers able to unwittingly spread the virus throughout life. Given that their blood is highly contagious and the virus can remain on surfaces or items for up to a week, chronically infected people provide a way for hepatitis B virus to hide in plain sight. Before routine vaccination, 9,000 children innocently got infected each year — that’s 9,000 reasons why hepatitis B vaccine recommendations are designed to protect children as early in life as possible.
My baby isn’t that baby: The argument against hepatitis B vaccine at birth

Historically, the main mode of transmission for hepatitis B in the U.S. was through sex; however, the opioid epidemic changed this. For example, a small study in 2019 found that about 60% of new cases resulted from injection drug use. Current estimates of cases due to sexual transmission are less than 4 in 10. But, because many people tend to focus on hepatitis B spread through sex and injection drug use, the birth dose recommendation for hepatitis B vaccine seems particularly unreasonable to some, so let’s take a step back and retrace the history of hepatitis B vaccine recommendations because the end result doesn’t tell the whole story.
The earliest recommendations
The hepatitis B vaccine was licensed in the U.S. in 1981, and the first vaccine recommendations were published in June 1982. Those recommendations targeted groups known to be at increased risk for hepatitis B, including healthcare providers, particularly those likely to be exposed to blood of infected patients; clients and staff at long-term care residencies for the developmentally disabled; patients getting hemodialysis and those with certain blood disorders; men who have sex with men; users of illegal injectable drugs; household and sexual contacts of those chronically infected with hepatitis B and those with acute cases of disease or recent needlestick injury exposures; classroom contacts of chronically infected individuals who may act aggressively or from whom exposure to their blood may be more likely; Alaskan natives, Pacific Islanders, and immigrants and refugees from areas with endemic disease; prison inmates; and infants born to hepatitis B-positive mothers.
Over time, a few other groups were added. In 1985, heterosexual people with multiple sexual partners and international travelers who were going to an area with endemic hepatitis B for more than six months were added to the recommended groups to be vaccinated. And in 1990, public safety workers likely to have contact with blood or blood-containing body fluids as family members of children adopted from countries with endemic hepatitis B were recommended to be vaccinated.
Vaccinating all infants
By 1991, an assessment of the impact of the hepatitis B recommendations aimed at high-risk individuals had demonstrated the strengths and weaknesses of that approach:
- The vaccine was preventing infections among people vaccinated before exposure, such as ethnic groups with high rates of childhood hepatitis B infections like Alaskan Natives where the rates had decreased 99% since introduction of the vaccine. Progress had also been made in decreasing infections among healthcare workers.
- The vaccine was also preventing infections among babies born to hepatitis B-positive mothers but determining which mothers to test had proven difficult. It was estimated that 35 to 65 of 100 hepatitis B-positive mothers were not being identified. As such in 1988, the recommendations were changed to test all mothers during pregnancy.
- Many high-risk teens and adults, however, remained unidentified or had already been infected, and conversely, many people diagnosed with a hepatitis B infection had no risk factors, so they never would have been identified even if the system was working well.
Cases were continuing to occur, and estimates at the time were that 1 million to 1.25 million people in the U.S. remained chronically infected and, therefore, could infect others. It was also clear that treatment would not eliminate the population of chronically infected individuals, which meant that prevention was even more important. First, vaccination would directly protect recipients, and second, fewer infections, especially during childhood, would translate to fewer chronically infected individuals and, therefore, less spread over time.
To decrease the likelihood of infection during childhood, all infants in the U.S. were recommended to be vaccinated against hepatitis B in 1991. Catch-up immunization campaigns followed in 1995 for all 11- to 12-year-olds not previously vaccinated against hepatitis B and in 1999 for all previously unvaccinated children up to 18 years of age.
Evolution of the birth dose
When a mother is determined to be hepatitis B positive, her baby is at much greater risk for hepatitis B infection. Often this occurs during birth when the baby is exposed to the mother’s blood, but it can also happen in the weeks and months after birth. In these instances, the baby’s chance of becoming infected can be significantly decreased if the baby receives a combination of vaccination and antibody at birth. As such, even the first set of hepatitis B vaccine recommendations in 1982 included these measures. However, because studies were ongoing related to the safety and effectiveness of the vaccine if given at birth, the 1982 recommendations indicated that the baby be vaccinated by 3 months of age. By 1991, when recommendations were changed to include hepatitis B vaccination of all infants, the studies looking at receipt of the vaccine within hours of birth had been completed without finding any concerns. As such, the updated recommendations indicated use of the birth dose for babies whose mothers were known to be hepatitis B positive and for those whose hepatitis B status was unknown. For babies whose mothers were known to be hepatitis B negative, the recommendation was to either get the dose before hospital discharge or at 1-2 months of age.
The success of targeting high-risk babies depends on both the mother’s hepatitis B status and the effectiveness of the medical system. First, if the mother has a high quantity of viral DNA in her blood, the baby may still be infected even if they received vaccine and antibody treatment at birth. If these DNA levels are known early enough, the mother can be treated with an antiviral medication beginning at 28 weeks of gestation. However, the success of these measures depends on the second factor — the effectiveness of the medical system. Specifically:
- Testing needs to be completed during pregnancy.
- The results need to be accurate and reported in a timely manner.
- The pregnant woman must not be exposed to the virus in the period between testing and delivery.
- The antiviral medication must be prescribed and taken by the hepatitis B-positive patients before delivery.
- The baby needs to receive the appropriate vaccination and antibody treatments upon birth. This includes following home births or other births that do not occur in hospital settings.
While these efforts may seem straightforward, successful implementation 100% of the time is not a given. As such, by 2002, the schedule was altered to recommend that all babies receive the hepatitis B vaccine at birth. This change was made understanding that the vaccine was safe and knowing that it would save some babies the fate of chronic hepatitis B when any part of the system failed.
The bottom line
Some babies of hepatitis B-positive mothers will remain untreated due to undiagnosed hepatitis B in their mother or because of medical system failures. Others will be exposed by household contacts or others whose infected blood they unwittingly encounter. Those infected early in life often develop chronic, undiagnosed hepatitis B, positioning them to unwittingly continue the spread of this virus — in day cares, on elementary school playgrounds, at sleepovers, and in sports leagues. By giving a birth dose of hepatitis B vaccine, all babies can begin to develop protection before any potential exposure to hepatitis B.
The proven impact of hepatitis B vaccine

Some have suggested that the birth dose of hepatitis B vaccine should be delayed in the U.S. As described above, this virus hides in plain sight. People don’t know they have it, so they don’t always know to take precautions to prevent spreading it. As such, each moment a child is without protection is another moment they may unwittingly be exposed. The U.S. is not unique in its recommendation for hepatitis B vaccine at birth. The World Health Organization (WHO) recommends universal vaccination of infants within 24 hours of birth, and country after country has reduced the impact of this disease using a birth dose. For example: Qatar has had a hepatitis B vaccine birth dose since 1989. Saudi Arabia since 1995. The Dominican Republic since 1997. Albania since 1998. In fact, as shown in blue on the map, in 2024 most countries had adopted the universal birth dose of hepatitis B vaccine.
Hepatitis B vaccines are safe
They have been extensively studied, and no causal associations were found for multiple conditions, including Guillain-Barré syndrome (GBS), multiple sclerosis (MS) or other conditions that cause demyelination of nerves, chronic fatigue syndrome, arthritis, autoimmune disorders, asthma, sudden infant death syndrome (SIDS), alopecia or diabetes.
Hepatitis B vaccines work
Between 1990 and 2015, use of hepatitis B vaccine in children around the globe was estimated to prevent 310 million new chronic hepatitis B infections. Between 2000 and 2019, hepatitis B vaccination of newborns and infants was estimated to prevent 22 million deaths globally from hepatitis B — about one death for every 13 vaccinations administered. Further, because deaths tend to occur decades after infection, it is estimated that between 2020 and 2030, another 16 million deaths will have been prevented by use of hepatitis B vaccine.
If hepatitis B vaccine was not available, about 5 of every 10 deaths from hepatitis B in a birth cohort (all those born in the same year) would be the result of infections acquired during early childhood.
The bottom line
If the U.S. removes or delays its birth dose recommendation as some have suggested, the chronic illnesses being experienced by today’s children would increase, not decrease, and the move would put the U.S. behind much of the rest of the world in terms of its approach to eliminating hepatitis B. Remember, before a hepatitis B vaccine was available in the U.S., about 9,000 children every year would be infected by an unknown source. If we begin sending our newborns home without their first dose of hepatitis B vaccine, those 9,000 annual cases will again begin accruing.
If hepatitis B virus was a superhero, its secret power would be its stealth. In fact, hepatitis B is so stealthy that many people don’t even know they are infected until decades after their initial exposure when they are diagnosed with liver disease.
Hepatitis B is a virus that replicates in liver cells. While many people recover after their initial infection, some go on to develop chronic hepatitis B. Many never know they were infected — or that they are so-called “carriers” — until they are diagnosed with hepatitis (inflammation of the liver), cirrhosis (severe liver damage) or liver cancer.
Many people consider hepatitis B an infection of only high-risk individuals, such as sex workers and injection drug users, but that viewpoint discounts hepatitis B’s superpower. Let’s explore more about how this virus can hide in plain sight and why that has led to the current vaccine recommendations to protect against hepatitis B.
There’s more to the spread of hepatitis B than meets the eye

Most people focus on the spread of hepatitis B through sex and injection drug use, but anyone can get hepatitis B, including children. Before hepatitis B vaccine was recommended for all children, each year about 18,000 infants and children were infected with the virus. About half of those children were infected during delivery or shortly thereafter because their mothers were infected. The other 9,000 often never knew when, where or how they were exposed to the virus. Three features contribute to hepatitis B’s stealthiness:
Invisible blood
While hepatitis B replicates in the liver, the virus, as well as proteins from the virus, circulate in the blood of infected individuals. This is how it is spread from person to person. Some people infected with hepatitis B virus have about 10 million to 1 billion hepatitis B virus particles in every milliliter of blood (a milliliter is about one-fifth of a teaspoon). But even among those with lower quantities (e.g., 100 to 1,000 virus particles per milliliter of blood), hepatitis B can be transmitted in amounts of blood not visible to the naked eye. Some body fluids have also been demonstrated to spread the virus, likely because of undetectable quantities of blood, including saliva, semen and vaginal fluids.
The presence of the virus in small or invisible amounts of blood means transmission can occur in unexpected ways or at places that people might not think about. For example:
- Although hepatitis B virus DNA and hepatitis B virus surface protein can be found in breast milk from mothers who are hepatitis B positive, a baby won’t be infected by the milk. However, if that mother has cracked or bleeding skin or nipples, the baby can be exposed to the virus while breastfeeding. In fact, the recommendation is that in this situation a hepatitis B-positive mother should not breastfeed until healed because of this likelihood.
- Hepatitis B can also be spread by other household contacts as well as people in day cares or schools and other non-household contacts. Examples include contact with blood from cuts in skin; pre-chewing of food; sharing gum or other foods; sharing toothbrushes, razors, utensils or other items that might contain undetectable amounts of blood, like washcloths or clothing; and contact with blood during sports or other physical activities.
Survival time
Hepatitis B virus is considered a hardy, or robust, virus, meaning it doesn’t die quickly once outside of the body. In fact, it can live for up to seven days on surfaces or objects that have been touched by an infected person’s blood or infected body fluids, including clothing, utensils, washcloths or bedding. People have also been infected in medical, dental, long-term care, and other facilities. These cases can occur from improperly sterilized medical equipment, needle sticks, or other exposures. For example, cases have resulted from acupuncture as well as tattooing and assisting with blood glucose monitoring.
Characteristics of infection
Two aspects of hepatitis B infections also contribute to the ease with which this virus can hide among us. First, only about 3 to 5 of every 10 older children and adults experience symptoms when they are initially exposed to the virus. In children less than 5 years of age, only 1 in 10 experience symptoms. As a result, it is easy for a person to be infected and not know. Second, the younger a person is when they are exposed, the more likely that they will become chronically infected. Fewer than 5 of every 100 healthy adults will become chronically infected, but about 3 of 10 children infected between 1 and 4 years of age and about 9 of 10 children infected during the first year of life will become chronically infected. Together these characteristics position the youngest children to unwittingly become chronically infected carriers. About 1 of every 4 people infected at birth or during early childhood will die earlier than others of the same age because of liver disease caused by hepatitis B.
The bottom line
In the U.S., about 4 in 5 people with chronic hepatitis B infections do not know they have this infection. They don’t have symptoms, and if they were exposed as children, they may be carriers able to unwittingly spread the virus throughout life. Given that their blood is highly contagious and the virus can remain on surfaces or items for up to a week, chronically infected people provide a way for hepatitis B virus to hide in plain sight. Before routine vaccination, 9,000 children innocently got infected each year — that’s 9,000 reasons why hepatitis B vaccine recommendations are designed to protect children as early in life as possible.
My baby isn’t that baby: The argument against hepatitis B vaccine at birth

Historically, the main mode of transmission for hepatitis B in the U.S. was through sex; however, the opioid epidemic changed this. For example, a small study in 2019 found that about 60% of new cases resulted from injection drug use. Current estimates of cases due to sexual transmission are less than 4 in 10. But, because many people tend to focus on hepatitis B spread through sex and injection drug use, the birth dose recommendation for hepatitis B vaccine seems particularly unreasonable to some, so let’s take a step back and retrace the history of hepatitis B vaccine recommendations because the end result doesn’t tell the whole story.
The earliest recommendations
The hepatitis B vaccine was licensed in the U.S. in 1981, and the first vaccine recommendations were published in June 1982. Those recommendations targeted groups known to be at increased risk for hepatitis B, including healthcare providers, particularly those likely to be exposed to blood of infected patients; clients and staff at long-term care residencies for the developmentally disabled; patients getting hemodialysis and those with certain blood disorders; men who have sex with men; users of illegal injectable drugs; household and sexual contacts of those chronically infected with hepatitis B and those with acute cases of disease or recent needlestick injury exposures; classroom contacts of chronically infected individuals who may act aggressively or from whom exposure to their blood may be more likely; Alaskan natives, Pacific Islanders, and immigrants and refugees from areas with endemic disease; prison inmates; and infants born to hepatitis B-positive mothers.
Over time, a few other groups were added. In 1985, heterosexual people with multiple sexual partners and international travelers who were going to an area with endemic hepatitis B for more than six months were added to the recommended groups to be vaccinated. And in 1990, public safety workers likely to have contact with blood or blood-containing body fluids as family members of children adopted from countries with endemic hepatitis B were recommended to be vaccinated.
Vaccinating all infants
By 1991, an assessment of the impact of the hepatitis B recommendations aimed at high-risk individuals had demonstrated the strengths and weaknesses of that approach:
- The vaccine was preventing infections among people vaccinated before exposure, such as ethnic groups with high rates of childhood hepatitis B infections like Alaskan Natives where the rates had decreased 99% since introduction of the vaccine. Progress had also been made in decreasing infections among healthcare workers.
- The vaccine was also preventing infections among babies born to hepatitis B-positive mothers but determining which mothers to test had proven difficult. It was estimated that 35 to 65 of 100 hepatitis B-positive mothers were not being identified. As such in 1988, the recommendations were changed to test all mothers during pregnancy.
- Many high-risk teens and adults, however, remained unidentified or had already been infected, and conversely, many people diagnosed with a hepatitis B infection had no risk factors, so they never would have been identified even if the system was working well.
Cases were continuing to occur, and estimates at the time were that 1 million to 1.25 million people in the U.S. remained chronically infected and, therefore, could infect others. It was also clear that treatment would not eliminate the population of chronically infected individuals, which meant that prevention was even more important. First, vaccination would directly protect recipients, and second, fewer infections, especially during childhood, would translate to fewer chronically infected individuals and, therefore, less spread over time.
To decrease the likelihood of infection during childhood, all infants in the U.S. were recommended to be vaccinated against hepatitis B in 1991. Catch-up immunization campaigns followed in 1995 for all 11- to 12-year-olds not previously vaccinated against hepatitis B and in 1999 for all previously unvaccinated children up to 18 years of age.
Evolution of the birth dose
When a mother is determined to be hepatitis B positive, her baby is at much greater risk for hepatitis B infection. Often this occurs during birth when the baby is exposed to the mother’s blood, but it can also happen in the weeks and months after birth. In these instances, the baby’s chance of becoming infected can be significantly decreased if the baby receives a combination of vaccination and antibody at birth. As such, even the first set of hepatitis B vaccine recommendations in 1982 included these measures. However, because studies were ongoing related to the safety and effectiveness of the vaccine if given at birth, the 1982 recommendations indicated that the baby be vaccinated by 3 months of age. By 1991, when recommendations were changed to include hepatitis B vaccination of all infants, the studies looking at receipt of the vaccine within hours of birth had been completed without finding any concerns. As such, the updated recommendations indicated use of the birth dose for babies whose mothers were known to be hepatitis B positive and for those whose hepatitis B status was unknown. For babies whose mothers were known to be hepatitis B negative, the recommendation was to either get the dose before hospital discharge or at 1-2 months of age.
The success of targeting high-risk babies depends on both the mother’s hepatitis B status and the effectiveness of the medical system. First, if the mother has a high quantity of viral DNA in her blood, the baby may still be infected even if they received vaccine and antibody treatment at birth. If these DNA levels are known early enough, the mother can be treated with an antiviral medication beginning at 28 weeks of gestation. However, the success of these measures depends on the second factor — the effectiveness of the medical system. Specifically:
- Testing needs to be completed during pregnancy.
- The results need to be accurate and reported in a timely manner.
- The pregnant woman must not be exposed to the virus in the period between testing and delivery.
- The antiviral medication must be prescribed and taken by the hepatitis B-positive patients before delivery.
- The baby needs to receive the appropriate vaccination and antibody treatments upon birth. This includes following home births or other births that do not occur in hospital settings.
While these efforts may seem straightforward, successful implementation 100% of the time is not a given. As such, by 2002, the schedule was altered to recommend that all babies receive the hepatitis B vaccine at birth. This change was made understanding that the vaccine was safe and knowing that it would save some babies the fate of chronic hepatitis B when any part of the system failed.
The bottom line
Some babies of hepatitis B-positive mothers will remain untreated due to undiagnosed hepatitis B in their mother or because of medical system failures. Others will be exposed by household contacts or others whose infected blood they unwittingly encounter. Those infected early in life often develop chronic, undiagnosed hepatitis B, positioning them to unwittingly continue the spread of this virus — in day cares, on elementary school playgrounds, at sleepovers, and in sports leagues. By giving a birth dose of hepatitis B vaccine, all babies can begin to develop protection before any potential exposure to hepatitis B.
The proven impact of hepatitis B vaccine

Some have suggested that the birth dose of hepatitis B vaccine should be delayed in the U.S. As described above, this virus hides in plain sight. People don’t know they have it, so they don’t always know to take precautions to prevent spreading it. As such, each moment a child is without protection is another moment they may unwittingly be exposed. The U.S. is not unique in its recommendation for hepatitis B vaccine at birth. The World Health Organization (WHO) recommends universal vaccination of infants within 24 hours of birth, and country after country has reduced the impact of this disease using a birth dose. For example: Qatar has had a hepatitis B vaccine birth dose since 1989. Saudi Arabia since 1995. The Dominican Republic since 1997. Albania since 1998. In fact, as shown in blue on the map, in 2024 most countries had adopted the universal birth dose of hepatitis B vaccine.
Hepatitis B vaccines are safe
They have been extensively studied, and no causal associations were found for multiple conditions, including Guillain-Barré syndrome (GBS), multiple sclerosis (MS) or other conditions that cause demyelination of nerves, chronic fatigue syndrome, arthritis, autoimmune disorders, asthma, sudden infant death syndrome (SIDS), alopecia or diabetes.
Hepatitis B vaccines work
Between 1990 and 2015, use of hepatitis B vaccine in children around the globe was estimated to prevent 310 million new chronic hepatitis B infections. Between 2000 and 2019, hepatitis B vaccination of newborns and infants was estimated to prevent 22 million deaths globally from hepatitis B — about one death for every 13 vaccinations administered. Further, because deaths tend to occur decades after infection, it is estimated that between 2020 and 2030, another 16 million deaths will have been prevented by use of hepatitis B vaccine.
If hepatitis B vaccine was not available, about 5 of every 10 deaths from hepatitis B in a birth cohort (all those born in the same year) would be the result of infections acquired during early childhood.
The bottom line
If the U.S. removes or delays its birth dose recommendation as some have suggested, the chronic illnesses being experienced by today’s children would increase, not decrease, and the move would put the U.S. behind much of the rest of the world in terms of its approach to eliminating hepatitis B. Remember, before a hepatitis B vaccine was available in the U.S., about 9,000 children every year would be infected by an unknown source. If we begin sending our newborns home without their first dose of hepatitis B vaccine, those 9,000 annual cases will again begin accruing.