The Future of Personalized Medicine is Here: KJ’s Story
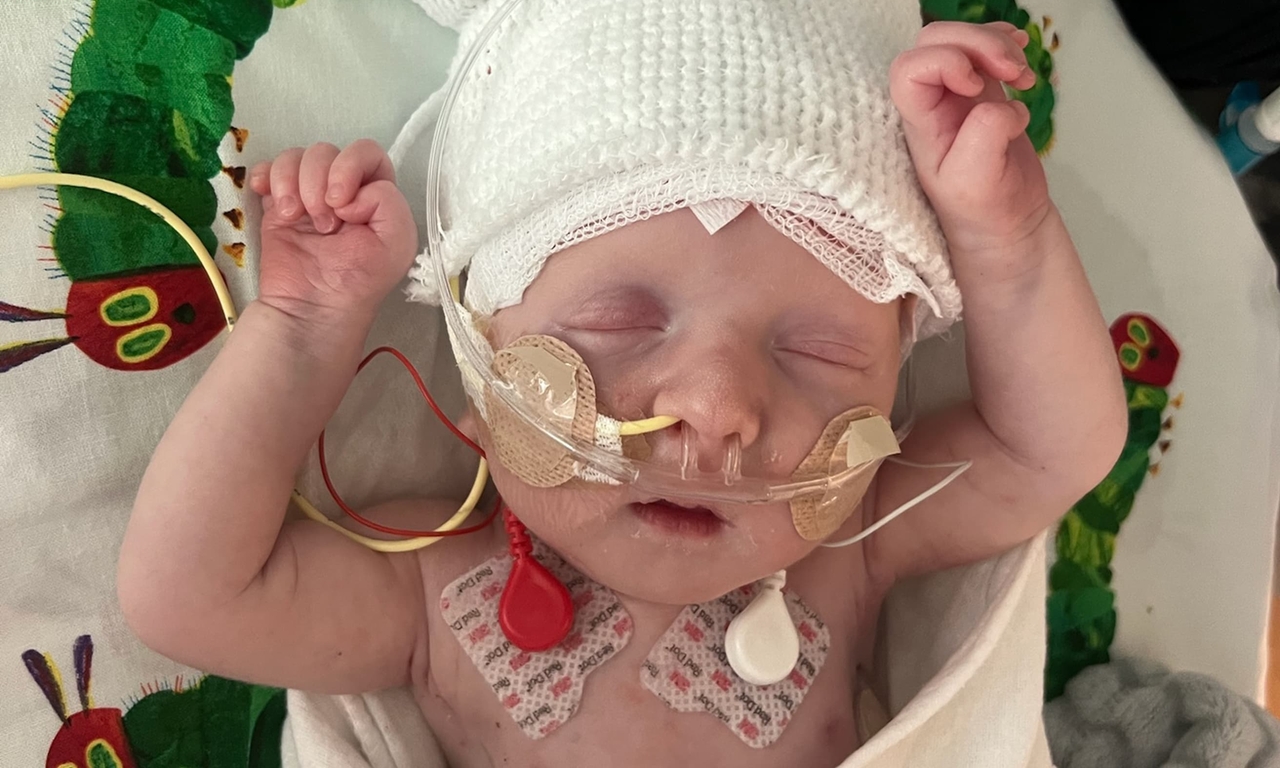
KJ was just 2 days old when a doctor at the Hospital of the University of Pennsylvania (Penn) noticed something was wrong. Though the baby seemed healthy at birth, he’d become unusually lethargic, wasn’t eating and struggled to maintain his temperature.
The doctor checked KJ’s blood ammonia level – which can be a marker for some metabolic diseases – and found it was extremely high. Doctors told KJ’s parents, Kyle and Nicole, that their son was very sick. But, they added, the best place for him was right next door at Children’s Hospital of Philadelphia (CHOP).
A rare diagnosis: urea cycle disorder
KJ’s first few days at CHOP were a whirlwind of tests, examinations and lots of questions. A team of experts from the Metabolic Disease Program and Division of Human Genetics diagnosed KJ with a rare urea cycle disorder.
Urea cycle disorders are genetic conditions caused by deficiencies in specific enzymes and lead to a toxic buildup of ammonia in the body. Left unchecked, the toxic accumulation can be fatal. Thankfully, CHOP is a leader in diagnosing and treating urea cycle disorders and has been actively researching new cell and gene therapies to treat them more effectively.
Doctors immediately placed KJ on dialysis to filter the ammonia out of his blood and stabilize his health. “He was small, but he was stubborn,” Nicole said. Kyle added, “He was a fighter from the start.”
Within a day of KJ’s arrival at CHOP, doctors ordered an innovative genomic test developed at CHOP to help infants in the ICU get rapid and impactful results. The testing, part of CHOP’s Baby Eagle Program, sequences a patient’s entire genome, then analyzes about 3,500 genes linked to genetic conditions that can present early in life. Knowing which gene or enzyme is affected is crucial for doctors to make informed decisions about treatment.
Two days later, KJ’s team discovered his disorder was a carbamoyl-phosphate synthase 1 deficiency (CPS1), caused by the CPS1 gene.
At that time, the only lifesaving treatment for CPS1 deficiency was a liver transplant, which is rare and often better suited to children who are older, larger and in better health. Nonetheless, KJ was added to the National Transplant Waiting List with the hope he would be stable enough for a liver transplant when – or if – a suitable organ became available.
“I had bought a Jalen Hurts jersey for KJ when he was first born,” Kyle said, a life-long Philadelphia Eagles fan. “But that moment – talking to the doctors – it hit me, he might not ever wear that jersey.”
Adds Nicole: “When we Googled CPS1 deficiency, we saw there were two likely outcomes – a liver transplant or death. We were in shock.”
While the family from Delaware County, PA, initially struggled to accept their son’s diagnosis, they quickly dove headfirst into learning everything they could about it. They worked hard to become KJ’s advocates and active members of his care team. The family decided KJ should remain in the hospital where he could receive constant support and monitoring around the clock.
KJ’s family spent countless hours at his bedside – talking to him, singing, reading and cuddling him. With help from a large support network, Nicole and Kyle rose to the challenges families with very sick children so often face: They provided care for KJ at the hospital, while also working and supporting their three other children at home in Clifton Heights.
Through it all, the family held onto hope that KJ would come home one day.
Developing a custom treatment
Before KJ was even born, a team of scientists at CHOP and the University of Pennsylvania (Penn) were researching how to use gene editing to create customized treatments for diseases like CPS1 deficiency.

Leading the group were Rebecca Ahrens-Nicklas, MD, PhD, a pediatric geneticist and Director of CHOP’s Gene Therapy for Inherited Metabolic Disorders Program, and Kiran Musunuru, MD, PhD, MPH, ML, MRA, a cardiologist, geneticist and gene editor at Penn.
“We’ve been practicing developing similar personalized therapies for about two years now with the idea that someday we might be in a position where we could very rapidly try to figure out how to use gene editing to correct a patient’s broken gene that’s responsible for their disease,” Dr. Musunuru said.
Over those two years (and before KJ’s birth), the team had learned a great deal about ways to correct faulty genes. With each potential genetic change they considered for gene editing therapy, the time it took to find a personalized solution shortened.
KJ’s genetic change was the seventh that the team considered for a novel gene editing therapy. On the same week KJ was placed on the transplant list, Dr. Ahrens-Nicklas and Dr. Musunuru approached the family about the possibility of participating in a gene editing research study.
“My biggest fear in all of this was giving false hope to a family,” Dr. Ahrens-Nicklas said. “But we got to a point where we thought there might actually be a clinical team and drug development team that could make a drug for KJ.”
Nicole and Kyle were open to discussing alternate treatments for KJ – even ones they didn’t fully understand – anything that might give him a better chance.
“The way I understood gene editing was like writing a sentence on paper. When you misspell a word, you go back in and rewrite it to spell it correctly,” Nicole said. “The gene editing would delete the mutated genes from his DNA and replace them with ones that worked properly.”
After months of research and successful results in the lab, as well as significant support from the National Institutes of Health and industry partners, the CHOP-Penn team developed a specialized therapy for KJ’s specific genetic disorder. The team offered the one-of-a-kind treatment to KJ’s family.
Launching hope, managing expectations
Dr. Ahrens-Nicklas and Dr. Musunuru were upfront and direct when talking to KJ’s parents. The therapy their team had developed was new and had never been tried before. They were cautiously optimistic, but there were no guarantees.

Kyle and Nicole said they understood the risks. “Our child was sick. We either had to have a liver transplant or give him this medicine that’s never been given to anybody before,” Kyle said. “It was an impossible choice, but we felt this was the best possible scenario for a life that, at one point, we didn’t know if he would be able to have.”
KJ’s parents agreed to the therapy and on Feb. 25, 2025, KJ became the first patient ever to receive a systemic personalized gene editing drug.
Read a news release about the historic gene editing therapy, developed by CHOP and Penn.
The drug was delivered by an infusion (in an IV line) and flowed into the bloodstream, traveling to the liver. KJ received the lowest possible dose of the therapy to allow his body time to adapt and minimize any risks.
“CRISPR, a gene editor, enters the nucleus of the cell,” Dr. Musunuru explained. “In this case, we programmed it to go to the site of the genetic variant that was causing the disease in KJ.”
The drug was specifically designed for KJ and the genetic variants he has. It’s personalized medicine.
Adds Dr. Ahrens-Nicklas: “The drug was specifically designed for KJ and the genetic variants he has. It’s personalized medicine.”
After the first treatment, KJ’s parents and entire medical team were cautiously optimistic.
“Nobody knows if it’s definitely going to work,” Kyle said after the first infusion, “but I think knowing that there’s so much hard work and so much love going into all this, it was important to us.”
Adds Nicole: “KJ’s a fighter. He’s proven that time and time again. We’re hoping this therapy brings him home.”
Moving ahead
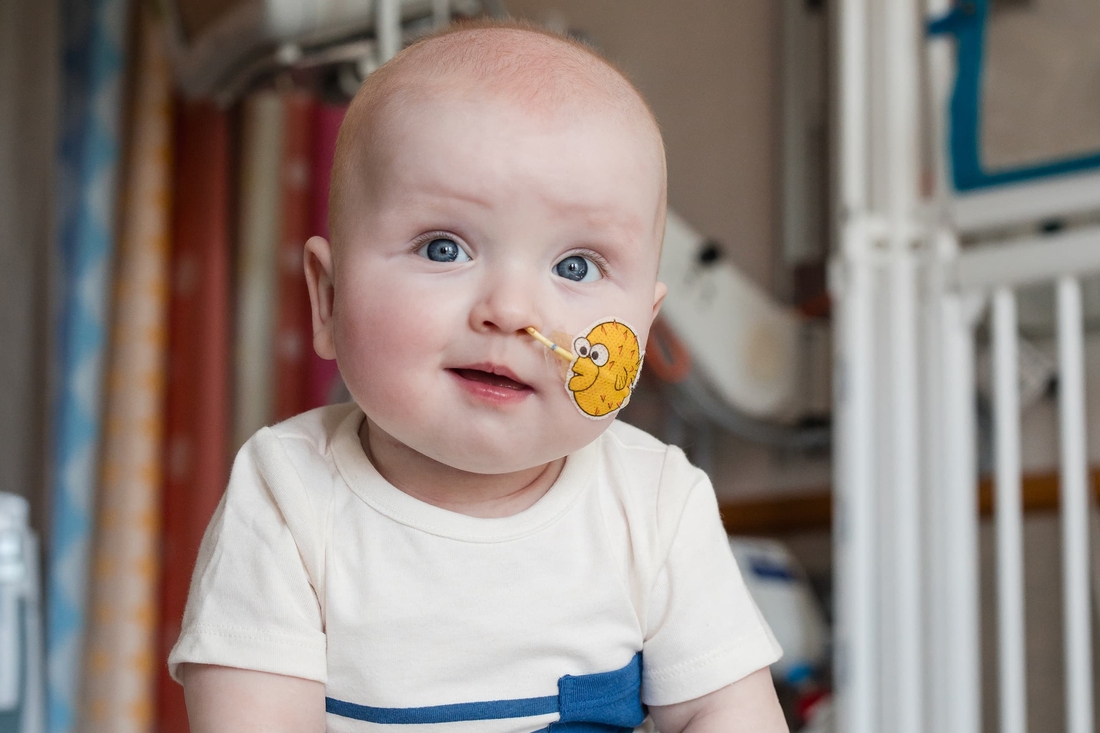
Even a few days after KJ’s first infusion, he showed signs of improvement. The color in his cheeks returned. He could tolerate more protein in his diet without causing a toxic increase in ammonia. And clinicians were able to slowly decrease his medication.
“He’s had quite a nice little growth spurt,” Dr. Ahrens-Nicklas said. “We know kids need protein to grow well. So, the fact that we’ve been able to give him more of the protein that he needs has really helped him grow some nice chubby cheeks.”
Over the next two months, KJ received two additional infusions at higher levels. Each treatment was administered safely, and he continues to be monitored as an inpatient at CHOP. He has not had any serious side effects to date and is now growing and thriving.
With each dose, KJ was better able to process the protein in his diet, leading to several positive milestones in his growth and development. He’s become more active, playful and more engaged with both his family and the hospital staff who support him.
While there are still many unknowns for KJ, doctors are hopeful the personalized gene editing has worked. Longer follow-up is needed to fully evaluate the benefits of this therapy.

“We’ve been operating in fight-or-flight mode for so long, that now it’s finally starting to look like the light at the end of the tunnel,” Nicole said. “When I look back at the little 4-pound peanut he was and now see this big, chunky, thriving baby, I’m so glad we were able to push him to show us what he could do and what he could become.
“I don’t think we’ll ever be able to put into words how grateful we are to CHOP for giving us this chance.”
Want to see more stories like this?
KJ is just one example of innovation, world-leading expertise and boundless compassion meeting to create a breakthrough. Discover more extraordinary stories of families whose lives were transformed at CHOP.
Your donation changes lives
Give today to the Gene Therapy for Inherited Metabolic Disorders Program to help find novel treatments for children like KJ (pictured) who are diagnosed with complex and life-threatening inherited metabolic disorders.
