Influenza/Flu Clinical Pathway — Emergency Department
Influenza/Flu Clinical Pathway — Emergency Department
Highly Pathogenic Avian Influenza, H5N1
Background
- Avian flu is widespread in wild birds worldwide
- There have been outbreaks in U.S. poultry and dairy cows
- Since spring 2024, there have been 67 human cases and 1 death in the U.S.
- There has been no human-to-human transmission documented to date
- Current risk to the general population remains low; however, providers should have a low threshold to test those with symptoms and exposures of risk
- PA Department of Public Health and the CDC have requested continued influenza A testing to monitor for novel influenza viruses, including H5N1, for awareness
Recommendations for Influenza A Testing
- All children admitted for respiratory symptoms and/or conjunctivitis
- Any children with respiratory symptoms and/or conjunctivitis and high-risk-exposure
- Quad or influenza A RNA PCR (NP or anterior nares swab)
- Consider conjunctival swab if clinical conjunctivitis, as save our specimen
- Avoid rapid flu antigen testing if H5N1 is of concern due to low sensitivity in the setting of a high-risk pathogen
- Note
Avian influenza is one of many subtypes of influenza A
PCR positive for influenza A is not likely to be avian flu - Call 5-SAFE if
High clinical concern/exposure, patients should isolate at home until testing results
PCR positive for influenza A with presence of any high-risk exposure - Patients with avian influenza or high-suspicion of avian influenza should be placed on expanded precautions
- AIIR room, N95, gown, gloves, and eye protection
Exposure Definition
For children who are Influenza A positive, review if any of the following Risk Factors are present in the preceding 10 days:
- Had contact with birds, poultry (including chickens), or dairy cows?
- Had contact with any wild or domestic non-pet animals including any birds (which includes home chickens) or cows in their enclosures?
- Participated in hunting birds (e.g., duck or geese hunting)?
- Had contact with outdoor cats that have recently been ill or died?
- Consumed raw or unpasteurized milk?
- Consumed or handled raw meat-based pet food?
Recommendations for Subtyping
If yes to any of the above questions, contact IP&C at 5-SAFE to help coordinate subtyping with the PA State Bureau of Labs (BOL).
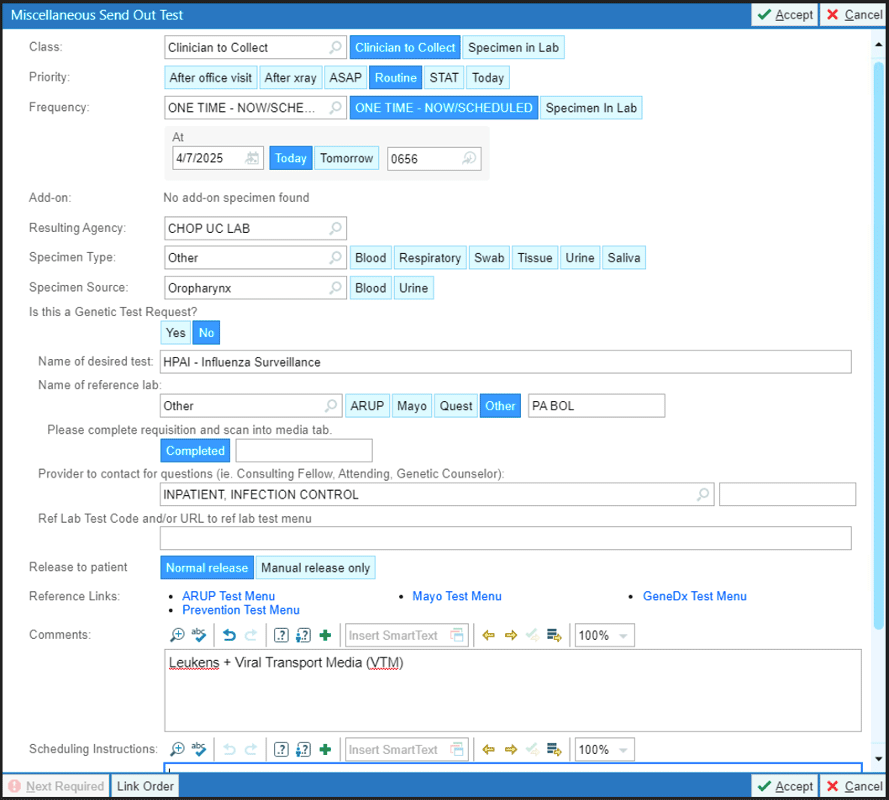
- Order miscellaneous send out lab which will be run on existing respiratory specimen.
- Enter “HPAI – Influenza Surveillance” for Name of Desired Test.
- Send nasopharyngeal (NP) or anterior nares and oropharyngeal (OP) swabs for Influenza A PCR (QUAD or single pathogen).
- Each specimen should be sent in a separate viral transport media (VTM).
- If conjunctivitis is present, a conjunctival swab should be collected from both eyes , even in children with single-eye involvement, and must be sent as a miscellaneous send-out to the Pennsylvania State Bureau of Labs for testing along with the above specimens. Both of the swabs should be placed in a single VTM.
- Requisition form required for each source (NP/anterior nares, OP and conjunctival if conjunctivitis present).
- IP&C will provide Medical Director name and contact information.
All Patients Under Investigation (PUIs) should be started on oseltamivir awaiting results of
the subtyping.
Epic Order for Miscellaneous Send Out Test
© 2025 Epic Systems Corporation
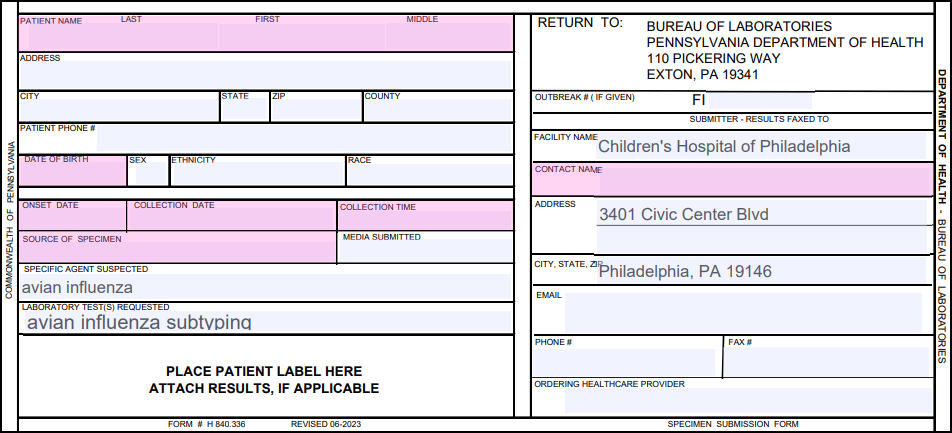
Order Requisition Form
Requisition form required for each source (NP/anterior nares, OP and conjunctival if conjunctivitis present).
© 2025 Epic Systems Corporation
Additional Resources
- PA DOH Health Alert June 2024
- Additional CDC Guidance for health care providers
- Accelerated Subtyping of Influenza A in Hospitalized Patients