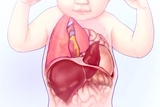
Congenital diaphragmatic hernia (CDH) occurs in about 1 in roughly 2,500 live births. At Children’s Hospital of Philadelphia (CHOP), we see nearly 50 babies with CDH each year. CDH is associated with significant morbidity and mortality related to the degree of pulmonary hypoplasia, the size of the diaphragmatic defect, pulmonary hypertension, and any other associated anomalies. CHOP’s experienced, multidisciplinary team helps to provide the best possible outcome for babies with CDH. This experience also means we are uniquely positioned to advance understanding into why CDH occurs and how it can be more effectively treated.
Seeking to understand the causes of CDH
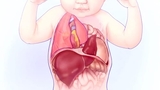
While the cause of CDH is still largely unknown, there is a strong genetic contribution. CDH can recur within families and/or occur as part of genetic syndromes or chromosomal disorders. CDH can be isolated, in which case the CDH is the only major difference in about 60% of individuals, or it can be syndromic, where CDH occurs with additional differences, or as part of a recognized syndrome.
Genetic differences — including chromosomal anomalies, copy number variants and sequence variants — are identified in ~30% of patients with CDH. The most common syndromic causes of CDH are Pallister-Killian syndrome, Fryns syndrome, Wolf-Hirschhorn syndrome, Cornelia de Lange syndrome, craniofrontal nasal syndrome, Donnai-Barrow syndrome, Simpson-Golabi-Behmel syndrome, CHARGE syndrome, Denys-Drash syndrome, Apert syndrome, Beckwith-Wiedemann syndrome, and 22q11.2 deletion syndrome.
Additional associated chromosomal microdeletions include 15q26, 8p23.1, 8q23, 5p15.2, 16p11.2, 17q12 and 1q41-42. There is also a growing number of genes associated with CDH. Mutations in these genes affect diaphragm development and can have pleiotropic effects on pulmonary and cardiac development.
However, despite dramatic advances in genetic diagnostic capabilities, a genetic etiology is not found in approximately 70% of individuals with CDH, including most with isolated CDH. Even with the increasing number of single genes found to be associated with CDH, the yield in looking for single-gene causes of isolated CDH remains low. It is more likely that genetic contributors to isolated CDH lie in regulatory elements, microRNAs, or other elements that control gene expression in the developing diaphragm.
At CHOP, Ian Krantz, MD, Director of the Roberts Individualized Medical Genetics Center (RIMGC) and Director of the Center for Cornelia de Lange Syndrome (CdLS) and Related Diagnoses, and his lab have characterized the molecular etiologies of two of the most common forms of syndromic CDH: CdLS and Pallister-Killian syndrome (PKS). We hope to use this experience and to leverage the broad scale genomic data generated by the CdLS and PKS studies to help elucidate the etiology of isolated CDH.
We see nearly 50 babies with CDH each year, more than any other center in the U.S. This experience makes us uniquely positioned to advance understanding into why CDH occurs and how it can be more effectively treated.
Defining underlying genetic etiologies of CDH
Using grant funding provided by the Fore Hadley Foundation, the RIMGC, in collaboration with the CDH program at the Center for Fetal Diagnosis and Treatment (CFDT), is trying to define the underlying genetic etiologies of CDH to better understand why CDH occurs.
Our Genomics of CDH study will establish and expand a clinical database and sample biorepository (collection of samples from blood, skin and diaphragm) of babies with CDH to perform innovative genetic testing including whole genome and RNA sequencing.
Our collaborative clinical and basic science team will use these samples to try to identify the genetic contributors to CDH. We hope that this will lead to improved diagnostics, counseling and management for families with a diagnosis of CDH.
By K. Taylor Wild, MD, Neonatal and Genetics Fellow
Featured in this article
Specialties & Programs
Congenital diaphragmatic hernia (CDH) occurs in about 1 in roughly 2,500 live births. At Children’s Hospital of Philadelphia (CHOP), we see nearly 50 babies with CDH each year. CDH is associated with significant morbidity and mortality related to the degree of pulmonary hypoplasia, the size of the diaphragmatic defect, pulmonary hypertension, and any other associated anomalies. CHOP’s experienced, multidisciplinary team helps to provide the best possible outcome for babies with CDH. This experience also means we are uniquely positioned to advance understanding into why CDH occurs and how it can be more effectively treated.
Seeking to understand the causes of CDH

While the cause of CDH is still largely unknown, there is a strong genetic contribution. CDH can recur within families and/or occur as part of genetic syndromes or chromosomal disorders. CDH can be isolated, in which case the CDH is the only major difference in about 60% of individuals, or it can be syndromic, where CDH occurs with additional differences, or as part of a recognized syndrome.
Genetic differences — including chromosomal anomalies, copy number variants and sequence variants — are identified in ~30% of patients with CDH. The most common syndromic causes of CDH are Pallister-Killian syndrome, Fryns syndrome, Wolf-Hirschhorn syndrome, Cornelia de Lange syndrome, craniofrontal nasal syndrome, Donnai-Barrow syndrome, Simpson-Golabi-Behmel syndrome, CHARGE syndrome, Denys-Drash syndrome, Apert syndrome, Beckwith-Wiedemann syndrome, and 22q11.2 deletion syndrome.
Additional associated chromosomal microdeletions include 15q26, 8p23.1, 8q23, 5p15.2, 16p11.2, 17q12 and 1q41-42. There is also a growing number of genes associated with CDH. Mutations in these genes affect diaphragm development and can have pleiotropic effects on pulmonary and cardiac development.
However, despite dramatic advances in genetic diagnostic capabilities, a genetic etiology is not found in approximately 70% of individuals with CDH, including most with isolated CDH. Even with the increasing number of single genes found to be associated with CDH, the yield in looking for single-gene causes of isolated CDH remains low. It is more likely that genetic contributors to isolated CDH lie in regulatory elements, microRNAs, or other elements that control gene expression in the developing diaphragm.
At CHOP, Ian Krantz, MD, Director of the Roberts Individualized Medical Genetics Center (RIMGC) and Director of the Center for Cornelia de Lange Syndrome (CdLS) and Related Diagnoses, and his lab have characterized the molecular etiologies of two of the most common forms of syndromic CDH: CdLS and Pallister-Killian syndrome (PKS). We hope to use this experience and to leverage the broad scale genomic data generated by the CdLS and PKS studies to help elucidate the etiology of isolated CDH.
We see nearly 50 babies with CDH each year, more than any other center in the U.S. This experience makes us uniquely positioned to advance understanding into why CDH occurs and how it can be more effectively treated.
Defining underlying genetic etiologies of CDH
Using grant funding provided by the Fore Hadley Foundation, the RIMGC, in collaboration with the CDH program at the Center for Fetal Diagnosis and Treatment (CFDT), is trying to define the underlying genetic etiologies of CDH to better understand why CDH occurs.
Our Genomics of CDH study will establish and expand a clinical database and sample biorepository (collection of samples from blood, skin and diaphragm) of babies with CDH to perform innovative genetic testing including whole genome and RNA sequencing.
Our collaborative clinical and basic science team will use these samples to try to identify the genetic contributors to CDH. We hope that this will lead to improved diagnostics, counseling and management for families with a diagnosis of CDH.
By K. Taylor Wild, MD, Neonatal and Genetics Fellow
Contact us
Richard D. Wood Jr. Center for Fetal Diagnosis and Treatment