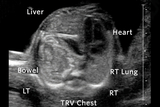
Congenital diaphragmatic hernia (CDH) is a developmental defect that causes respiratory complications in the first hours of life. Despite many advances in treatment of this condition after birth, the effects of CDH occur early in gestation and can result in pulmonary hypoplasia and pulmonary hypertension, both of which cause significant morbidity and mortality.
The Center for Fetal Diagnosis and Treatment at Children's Hospital of Philadelphia (CHOP) continues to lead translational and clinical outcomes research into prenatal and postnatal interventions to treat this complex birth defect. Here are details on some of those efforts.
Pharmacologic therapies
Our team has been studying the potential of prenatal pharmacologic therapies to improve lung and pulmonary vascular development in children with CDH. We recently evaluated the effect of prenatal retinoic acid (RA) on postnatal lung function, structure and pulmonary artery (PA) blood flow in the rat CDH model and found that RA accelerated lung maturation indicated by increased alveoli number and thinner interalveolar septa and was associated with decreased PA resistance and improved oxygenation.
Our researchers also recently evaluated the use of prenatal maternal dexamethasone (DM) and/or sildenafil in the nitrofen CDH rat model. We found that prenatal DM or sildenafil treatment increased pulmonary compliance and decreased pulmonary vascular resistance respectively. The treatment was associated with improved neonatal gas exchange but had a detrimental effect on lung and fetal growth.
- Burgos CM, Davey MG, Riley JS, Jia H, Flake AW, Peranteau WH. Lung function and pulmonary artery blood flow following prenatal maternal retinoic acid and imatinib in the nitrofen model of congenital diaphragmatic hernia. J Pediatr Surg. 2018;53(9):1681–1687.
- Burgos CM, Pearson EG, Davey M, Riley J, Jia H, Laje P, Flake AW, Peranteau WH. Improved pulmonary function in the nitrofen model of congenital diaphragmatic hernia following prenatal maternal dexamethasone and/ or sildenafil. Pediatr Res. 2016;80(4):577–585.
Gene therapy
A major limitation to adeno-associated virus (AAV) gene therapy is the generation of host immune responses to viral vector antigens and the transgene product. The ability to induce immune tolerance to foreign protein has the potential to overcome this host immunity. Acquisition and maintenance of tolerance to viral vector antigens and transgene products may also permit repeat administration thereby enhancing therapeutic efficacy. In utero gene transfer (IUGT) takes advantage of the immunologic immaturity of the fetus to induce immune tolerance to foreign antigens.
Our team has been engaged in preclinical large animal studies evaluating clinically relevant viral vectors that efficiently transduce airway epithelial cells. This research group also recently published a large animal study assessing transgene expression and postnatal immune tolerance to GFP and viral antigens in fetal sheep. We recently published a study demonstrating, in a preclinical large animal model, the potential of IUGT to achieve host immune tolerance to the viral vector transgene product. The study also suggests that a single exposure to the vector capsid proteins at the time of IUGT is inadequate to induce tolerance to viral vector antigens.
- McClain LE, Davey MG, Zoltick PW, Limberis MP, Flake AW, Peranteau WH. Vector serotype screening for use in ovine perinatal lung gene therapy. J Pediatr Surg. 2016;51(6):879–884.
- Davey MG, Riley JS, Andrews A, Tyminski A, Limberis M, Pogoriler JE, Partridge E, Olive A, Hedrick HL, Flake AW, Peranteau WH. Induction of immune tolerance to foreign protein via adeno-associated viral vector gene transfer in mid-gestation fetal sheep. PLoS One. 2017;12(1):e0171132.
Tracheal occlusion
Our team has spent nearly 20 years studying the effects of tracheal occlusion in CDH. We are currently involved in the TOTAL trial (Tracheal Occlusion To Accelerate Lung growth). In tracheal occlusion, a balloon is placed in the trachea of the fetus to stop lung fluid from flowing from the lungs to the amniotic cavity. This produces an increase in pulmonary pressure, which accelerates lung growth by inducing stretch. It has been found to increase postnatal survival and decrease the need for prolonged oxygen administration in babies with severe CDH.
Our team recently began studying the benefits of cyclical tracheal occlusion — in which the trachea is occluded in lambs for 48 hours with 1-hour release for a period of two weeks — as opposed to prolonged tracheal occlusion — in which the trachea is occluded for a period of weeks. To test this treatment, we are using a system we recently developed that incorporates a pumpless oxygenator circuit connected to the fetus of a lamb via an umbilical cord interface that is maintained within a closed ‘amniotic fluid’ circuit that closely reproduces the environment of the womb.
The device is currently in development to prepare for clinical trials in extreme preterm infants, but it has also proven extremely useful in studying the effects of prenatal treatments in animal models. Lambs that are developmentally equivalent to the extreme premature human infant can be physiologically supported in this extra-uterine device for up to four weeks.
If the cyclical approach shows benefit, our team plans to work with bioengineers to develop a smart balloon that can be inflated and deflated while the fetus is in its mother’s womb.
- Partridge EA, Davey MG, Hornick MA, McGovern PE, Mejaddam AY, Vrecenak JD, Mesas-Burgos C, Olive A, Caskey RC, Weiland TR, Han J, Schupper AJ, Connelly JT, Dysart KC, Rychik J, Hedrick HL, Peranteau WH, Flake AW. An extra-uterine system to physiologically support the extreme premature lamb. Nat Commun. 2017;8:15112.
Postnatal treatment
Extracorporeal membrane oxygenation (ECMO) is commonly required in neonates with CDH complicated by pulmonary hypertension. It can provide breathing and heart support to patients with life-threatening circulatory or respiratory failure for days to weeks while doctors treat their underlying illness. The ECMO Center at CHOP — named a Platinum Level Center of Excellence by the Extracorporeal Life Support Organization — is one of the most active in the country, having supported more than 1,300 patients since it was established in 1990. The program’s multidisciplinary team is comprised of pediatric surgeons, neonatologists, PICU intensivists, anesthesiologists, perfusionists, specially trained nurses and respiratory therapists.
Even in the hands of an expert team, ECMO carries significant risk, and is contraindicated in the setting of extreme prematurity or intracranial hemorrhage. Our researchers have been studying pumpless arteriovenous ECMO (P-ECMO) as an alternative for respiratory support in a lamb model of CDH. We believe it has the potential to bridge neonates with decompensated respiratory failure to CDH repair with no requirement for systemic anticoagulation, and may be applicable to patients currently precluded from conventional ECMO support.
- Partridge EA, Davey MG, Hornick M, Dysart KC, Olive A, Caskey R, Connelly JT, Hedrick HL, Peranteau WH, Flake AW. Pumpless arteriovenous extracorporeal membrane oxygenation: A novel mode of respiratory support in a lamb model of congenital diaphragmatic hernia. J Pediatr Surg. 2018;53(8):1453–1460.
Featured in this article
Specialties & Programs
Congenital diaphragmatic hernia (CDH) is a developmental defect that causes respiratory complications in the first hours of life. Despite many advances in treatment of this condition after birth, the effects of CDH occur early in gestation and can result in pulmonary hypoplasia and pulmonary hypertension, both of which cause significant morbidity and mortality.
The Center for Fetal Diagnosis and Treatment at Children's Hospital of Philadelphia (CHOP) continues to lead translational and clinical outcomes research into prenatal and postnatal interventions to treat this complex birth defect. Here are details on some of those efforts.
Pharmacologic therapies
Our team has been studying the potential of prenatal pharmacologic therapies to improve lung and pulmonary vascular development in children with CDH. We recently evaluated the effect of prenatal retinoic acid (RA) on postnatal lung function, structure and pulmonary artery (PA) blood flow in the rat CDH model and found that RA accelerated lung maturation indicated by increased alveoli number and thinner interalveolar septa and was associated with decreased PA resistance and improved oxygenation.
Our researchers also recently evaluated the use of prenatal maternal dexamethasone (DM) and/or sildenafil in the nitrofen CDH rat model. We found that prenatal DM or sildenafil treatment increased pulmonary compliance and decreased pulmonary vascular resistance respectively. The treatment was associated with improved neonatal gas exchange but had a detrimental effect on lung and fetal growth.
- Burgos CM, Davey MG, Riley JS, Jia H, Flake AW, Peranteau WH. Lung function and pulmonary artery blood flow following prenatal maternal retinoic acid and imatinib in the nitrofen model of congenital diaphragmatic hernia. J Pediatr Surg. 2018;53(9):1681–1687.
- Burgos CM, Pearson EG, Davey M, Riley J, Jia H, Laje P, Flake AW, Peranteau WH. Improved pulmonary function in the nitrofen model of congenital diaphragmatic hernia following prenatal maternal dexamethasone and/ or sildenafil. Pediatr Res. 2016;80(4):577–585.
Gene therapy
A major limitation to adeno-associated virus (AAV) gene therapy is the generation of host immune responses to viral vector antigens and the transgene product. The ability to induce immune tolerance to foreign protein has the potential to overcome this host immunity. Acquisition and maintenance of tolerance to viral vector antigens and transgene products may also permit repeat administration thereby enhancing therapeutic efficacy. In utero gene transfer (IUGT) takes advantage of the immunologic immaturity of the fetus to induce immune tolerance to foreign antigens.
Our team has been engaged in preclinical large animal studies evaluating clinically relevant viral vectors that efficiently transduce airway epithelial cells. This research group also recently published a large animal study assessing transgene expression and postnatal immune tolerance to GFP and viral antigens in fetal sheep. We recently published a study demonstrating, in a preclinical large animal model, the potential of IUGT to achieve host immune tolerance to the viral vector transgene product. The study also suggests that a single exposure to the vector capsid proteins at the time of IUGT is inadequate to induce tolerance to viral vector antigens.
- McClain LE, Davey MG, Zoltick PW, Limberis MP, Flake AW, Peranteau WH. Vector serotype screening for use in ovine perinatal lung gene therapy. J Pediatr Surg. 2016;51(6):879–884.
- Davey MG, Riley JS, Andrews A, Tyminski A, Limberis M, Pogoriler JE, Partridge E, Olive A, Hedrick HL, Flake AW, Peranteau WH. Induction of immune tolerance to foreign protein via adeno-associated viral vector gene transfer in mid-gestation fetal sheep. PLoS One. 2017;12(1):e0171132.
Tracheal occlusion
Our team has spent nearly 20 years studying the effects of tracheal occlusion in CDH. We are currently involved in the TOTAL trial (Tracheal Occlusion To Accelerate Lung growth). In tracheal occlusion, a balloon is placed in the trachea of the fetus to stop lung fluid from flowing from the lungs to the amniotic cavity. This produces an increase in pulmonary pressure, which accelerates lung growth by inducing stretch. It has been found to increase postnatal survival and decrease the need for prolonged oxygen administration in babies with severe CDH.
Our team recently began studying the benefits of cyclical tracheal occlusion — in which the trachea is occluded in lambs for 48 hours with 1-hour release for a period of two weeks — as opposed to prolonged tracheal occlusion — in which the trachea is occluded for a period of weeks. To test this treatment, we are using a system we recently developed that incorporates a pumpless oxygenator circuit connected to the fetus of a lamb via an umbilical cord interface that is maintained within a closed ‘amniotic fluid’ circuit that closely reproduces the environment of the womb.
The device is currently in development to prepare for clinical trials in extreme preterm infants, but it has also proven extremely useful in studying the effects of prenatal treatments in animal models. Lambs that are developmentally equivalent to the extreme premature human infant can be physiologically supported in this extra-uterine device for up to four weeks.
If the cyclical approach shows benefit, our team plans to work with bioengineers to develop a smart balloon that can be inflated and deflated while the fetus is in its mother’s womb.
- Partridge EA, Davey MG, Hornick MA, McGovern PE, Mejaddam AY, Vrecenak JD, Mesas-Burgos C, Olive A, Caskey RC, Weiland TR, Han J, Schupper AJ, Connelly JT, Dysart KC, Rychik J, Hedrick HL, Peranteau WH, Flake AW. An extra-uterine system to physiologically support the extreme premature lamb. Nat Commun. 2017;8:15112.
Postnatal treatment
Extracorporeal membrane oxygenation (ECMO) is commonly required in neonates with CDH complicated by pulmonary hypertension. It can provide breathing and heart support to patients with life-threatening circulatory or respiratory failure for days to weeks while doctors treat their underlying illness. The ECMO Center at CHOP — named a Platinum Level Center of Excellence by the Extracorporeal Life Support Organization — is one of the most active in the country, having supported more than 1,300 patients since it was established in 1990. The program’s multidisciplinary team is comprised of pediatric surgeons, neonatologists, PICU intensivists, anesthesiologists, perfusionists, specially trained nurses and respiratory therapists.
Even in the hands of an expert team, ECMO carries significant risk, and is contraindicated in the setting of extreme prematurity or intracranial hemorrhage. Our researchers have been studying pumpless arteriovenous ECMO (P-ECMO) as an alternative for respiratory support in a lamb model of CDH. We believe it has the potential to bridge neonates with decompensated respiratory failure to CDH repair with no requirement for systemic anticoagulation, and may be applicable to patients currently precluded from conventional ECMO support.
- Partridge EA, Davey MG, Hornick M, Dysart KC, Olive A, Caskey R, Connelly JT, Hedrick HL, Peranteau WH, Flake AW. Pumpless arteriovenous extracorporeal membrane oxygenation: A novel mode of respiratory support in a lamb model of congenital diaphragmatic hernia. J Pediatr Surg. 2018;53(8):1453–1460.
Contact us
Richard D. Wood Jr. Center for Fetal Diagnosis and Treatment