
At two-months-old, Rahemeen Nabeel was diagnosed with beta thalassemia, an inherited blood disorder that affects the production of normal hemoglobin, a protein in the red blood cells that carries oxygen to tissues throughout the body. The disorder caused by mutations in a gene called beta-globin. Rahemeen has a severe form of the disease, which required blood transfusions every two to five weeks in the hospital.
“Rahemeen required frequent monitoring for complications caused by the disease as well as transfusions, which can cause iron to accumulate in the liver, spleen, heart and other organs,” said Janet Kwiatkowski, MD, MSCE, Director of the Thalassemia Center and Co-Director of the Sickle Cell and Red Cell Disorders Curative Therapy Center (CuRED) at Children’s Hospital of Philadelphia (CHOP).
Rahemeen and her family were living in Dubai when she was diagnosed. In search for better treatment options and a possible stem cell donor (which was the only curative treatment at the time), the family traveled across the globe: Paris, Rome, Switzerland, Turkey, and Korea. They moved to the United States and settled in New Jersey, where they found answers at CHOP.
CHOP was a leading study site for a brand-new treatment for beta-thalassemia: gene therapy. After years of clinical trials conducted at CHOP and other sites, in August 2022, the U.S. Food and Drug Administration (FDA) approved the first gene therapy for transfusion-dependent beta thalassemia called Zynteglo®. CHOP was the first Qualified Treatment Center offering the therapy, and Rahemeen was the first patient outside a clinical trial to receive it.
Gene therapy corrects the genetic problem that causes beta thalassemia. The process involves collecting stem cells from the patient and sending the cells to the lab, where a working copy of the dysfunctional gene is added to the cells. Once the modified stem cells are ready, they are sent back to the hospital where the patient will be admitted to receive the gene therapy.
“Rahemeen had to undergo a round of chemotherapy before receiving her own modified stem cells through an IV infusion,” said Timothy S. Olson, MD, PhD, Medical Director of the Cellular Therapy and Transplant Section and Co-Director of CuRED at CHOP. “I’m happy to share that the therapy has transformed Rahemeen’s life. She no longer needs any blood transfusions nor does she have any recurring pain, and she consistently has high energy.”
In April 2023, Rahemeen received her modified cells under the care of Dr. Olson. After a 40-day hospital stay, Rahemeen was on the road to a full recovery. Today, the 11-year-old sees her possibilities as endless.
“I’m going to try out for soccer! And I also have time now to go to trampoline parks and dance parties, said Rahemeen. “You’re free to do everything now!”
CHOP captured Rahemeen’s inspiring journey as part of its CHOP Stories video series to share this experience with future patients and the world. You can watch her story, and others, here.
Featured in this article
Experts
Specialties & Programs
At two-months-old, Rahemeen Nabeel was diagnosed with beta thalassemia, an inherited blood disorder that affects the production of normal hemoglobin, a protein in the red blood cells that carries oxygen to tissues throughout the body. The disorder caused by mutations in a gene called beta-globin. Rahemeen has a severe form of the disease, which required blood transfusions every two to five weeks in the hospital.
“Rahemeen required frequent monitoring for complications caused by the disease as well as transfusions, which can cause iron to accumulate in the liver, spleen, heart and other organs,” said Janet Kwiatkowski, MD, MSCE, Director of the Thalassemia Center and Co-Director of the Sickle Cell and Red Cell Disorders Curative Therapy Center (CuRED) at Children’s Hospital of Philadelphia (CHOP).
Rahemeen and her family were living in Dubai when she was diagnosed. In search for better treatment options and a possible stem cell donor (which was the only curative treatment at the time), the family traveled across the globe: Paris, Rome, Switzerland, Turkey, and Korea. They moved to the United States and settled in New Jersey, where they found answers at CHOP.
CHOP was a leading study site for a brand-new treatment for beta-thalassemia: gene therapy. After years of clinical trials conducted at CHOP and other sites, in August 2022, the U.S. Food and Drug Administration (FDA) approved the first gene therapy for transfusion-dependent beta thalassemia called Zynteglo®. CHOP was the first Qualified Treatment Center offering the therapy, and Rahemeen was the first patient outside a clinical trial to receive it.
Gene therapy corrects the genetic problem that causes beta thalassemia. The process involves collecting stem cells from the patient and sending the cells to the lab, where a working copy of the dysfunctional gene is added to the cells. Once the modified stem cells are ready, they are sent back to the hospital where the patient will be admitted to receive the gene therapy.
“Rahemeen had to undergo a round of chemotherapy before receiving her own modified stem cells through an IV infusion,” said Timothy S. Olson, MD, PhD, Medical Director of the Cellular Therapy and Transplant Section and Co-Director of CuRED at CHOP. “I’m happy to share that the therapy has transformed Rahemeen’s life. She no longer needs any blood transfusions nor does she have any recurring pain, and she consistently has high energy.”
In April 2023, Rahemeen received her modified cells under the care of Dr. Olson. After a 40-day hospital stay, Rahemeen was on the road to a full recovery. Today, the 11-year-old sees her possibilities as endless.
“I’m going to try out for soccer! And I also have time now to go to trampoline parks and dance parties, said Rahemeen. “You’re free to do everything now!”
CHOP captured Rahemeen’s inspiring journey as part of its CHOP Stories video series to share this experience with future patients and the world. You can watch her story, and others, here.
Recommended reading
A Life-changing Therapy for a Devastating Disease
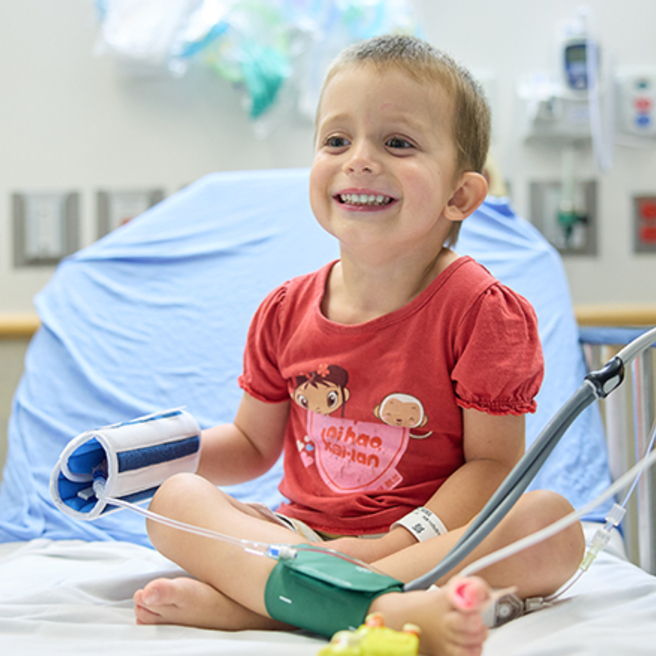
FDA approves first potentially curative gene therapy for the rare blood disorder beta thalassemia.
Patients at CHOP Begin Newly Approved Sickle Cell Gene Therapies
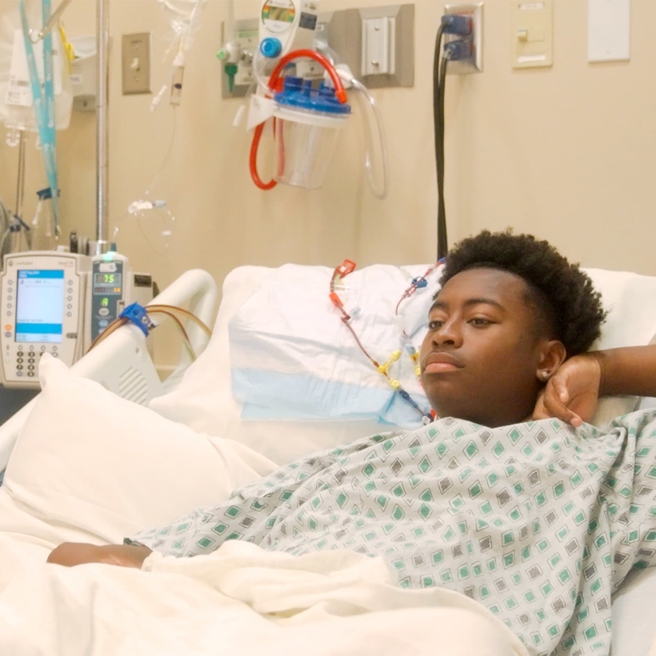
CHOP announced that its first patients outside of clinical trials have begun the treatment process for the newly FDA approved sickle cell gene therapies.
Contact us
Jennifer Lee
Sickle Cell and Red Cell Disorders Curative Therapy Center (CuRED)