What is a congenital cystic adenomatoid malformation (CCAM/CPAM)?
Congenital cystic adenomatoid malformation (CCAM) is a benign lung lesion that appears before birth as a cyst or mass in the chest. It is made up of abnormal lung tissue that does not function properly, but continues to grow. CCAM is also frequently referred to as a congenital pulmonary airway malformation (CPAM).
CCAM/CPAM is the most common type of fetal lung lesion. It develops before a baby is born, and can vary in size and be either fluid-filled or solid. A large cyst is called a macrocystic lesion, and a small cyst or solid appearing lesion is called microcystic.
Some CCAM/CPAMs can be life-threatening if they are not treated, so early and accurate diagnosis is important. The vast majority of CCAM/CPAM lesions are small enough that they will not cause any problems to the baby during pregnancy and the CCAM/CPAM can be removed after birth. However, some large lesions can cause serious and even fatal complications, including fetal heart failure (also called fetal hydrops) or maternal mirror syndrome. These cases may require treatment before birth.
Types of CCAM/CPAM
These images illustrate different types of CCAM/CPAM, and how the different sizes and presentations of lung lesions can impact the heart and lungs.
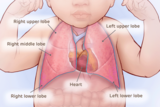
Normal heart and lung anatomy with lobes of lungs
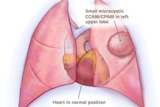
Small microcystic CCAM/CPAM heart not compressed
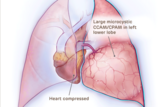
Large microcystic CCAM/CPAM heart compressed
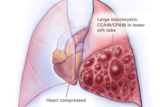
Large macrocystic CCAM/CPAM heart compressed
Causes of CCAM/CPAM
The causes of CCAM/CPAM are unknown. It’s not linked to a gene or to a chromosomal abnormality, and does not run in families (is not hereditary). The malformation results from abnormal lung tissue that grows, usually in one lobe of the lung.
Signs and symptoms of CCAM/CPAM
Congenital cystic adenomatoid malformations are typically discovered during a routine prenatal ultrasound. The ultrasound technician may notice a bright spot in your baby’s lung that indicates a cyst or mass.
CCAM/CPAMs typically increase in size until 26 – 28 weeks’ gestation, after which the size of many CCAM/CPAMs plateau or even decrease.
Evaluation and diagnosis of CCAM/CPAM
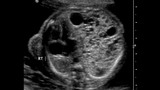
A CCAM/CPAM is typically diagnosed by prenatal ultrasound. If you are referred to CHOP’s Center for Fetal Diagnosis and Treatment, you will have a comprehensive one-day evaluation. More than 3,013 patients with suspected lung lesions have been referred to our team, experience that makes us uniquely equipped to evaluate and manage your child’s condition.
Our team of dedicated fetal radiologists use the most advanced prenatal imaging techniques and state-of-the-art technologies available to gather detailed information about your baby’s diagnosis.
- You will have a high-resolution fetal ultrasound to evaluate fetal anatomy as well as the lung lesion.
- A dedicated fetal cardiologist will perform a fetal echocardiogram to evaluate heart function and structure.
- If indicated, you may also have an ultrafast fetal MRI, pioneered at CHOP, to provide additional anatomic detail about the lung lesion.
These specialized tests help us determine the type, size and location of the mass, where the blood supply is coming from, and allow us to see any impact it may be having on your baby’s heart.
Depending on gestational age of your baby and the size of the mass, you will continue to have ultrasounds once or twice a week to closely monitor the growth of the lung lesion.
Determining severity of CCAM/CPAM before delivery
Using a special measurement called a CCAM volume ratio, or CVR, we will carefully measure the size of the lung lesion in relation to the size of your baby (based on their head circumference). The CCAM volume ratio was developed by experts at Children’s Hospital of Philadelphia. It is the most accurate way of following the growth of the CCAM/CPAM over time, and helps us determine the best course of treatment for your baby.
If the CVR is more than 1.6, your baby is at higher risk for developing heart failure. In these cases, prenatal intervention may be needed.
The lower the CVR, the better. But continued monitoring is important to make sure the volume of the lesion doesn’t increase and require treatment before birth.
Management of CCAM/CPAM during pregnancy
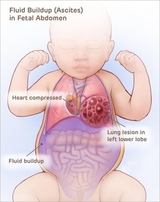
Most CCAM/CPAMs are small and do not cause any stress on your baby’s heart before birth, or any problems with breathing after birth. These small CCAM/CPAMs only require close monitoring before birth to ensure they remain small.
In rare instances, CCAM/CPAMs grow to be large and may require treatment before birth. When the lung mass grows, it can take up valuable space in the chest. This can restrict normal lung growth and can lead to underdeveloped lungs which will not function adequately at birth. Large lesions can also shift the heart and impair blood flow. This can lead to fetal heart failure (fetal hydrops) and cause the buildup of fluid in the fetus and placenta.
If the fetus develops hydrops, you may "mirror" your baby’s illness. If you are carrying a baby diagnosed with CCAM/CPAM, your pregnancy must be carefully monitored for high blood pressure and other signs and symptoms of the maternal mirror syndrome, or preeclampsia. Blood pressure and urine tests can monitor for signs of preeclampsia.

Why choose us for CCAM/CPAM care
CHOP provides comprehensive care for both mother and baby diagnosed with fetal lung lesions. From before birth to long-term follow-up, we are here for you every step of the way.
Fetal intervention for CCAM or CPAM
For these large CCAM/CPAMs, we have a number of different treatment options before birth to decrease the size of the CCAM/CPAM and reduce the chance of fetal heart failure. These treatment options depend on the type of CCAM/CPAM (microcystic or macrocystic) and the gestational age of your fetus. These options be discussed with you at the time of your evaluation.
- Steroids to prevent growth of lesion: Solid microcystic lesions sometimes respond to steroids, which may be used to interrupt the growth of the lesion. Using steroids to slow the growth of the CCAM/CPAM can prevent the need for fetal surgery in certain cases. (Read a recent study from our team about the use of steroids in managing fetal lung lesions.)
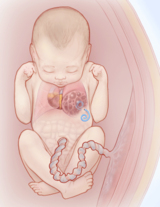
Fluid-filled macrocystic lesions may be drained with a shunt placed into the fetal chest, diverting fluid from the cyst to the amniotic sac. © CHOP/CFDT Draining fluid from the cyst: Fluid-filled macrocystic lesions can be drained prenatally to remove fluid and reduce the size of the mass. The fluid may reaccumulate, in which case a shunt can be placed. The shunting procedure itself is performed under ultrasound guidance. A large trocar (hollow needle) is guided through the mother’s abdomen and uterus, and into the fetal chest. The shunt is passed through the trocar into the fluid-filled cyst. The goal is to divert fluid from the cyst to the amniotic sac. The shunt will remain until delivery. The goal of these procedures is to decrease the size of the mass to ward off heart failure (fetal hydrops).
- Open fetal surgery to remove CCAM/CPAM: We have learned that open fetal surgery is now rarely necessary due to advances in care before birth. However, if your baby is not responding to other prenatal treatments and showing early signs of heart failure (hydrops), our experienced team is prepared to remove the CCAM/CPAM before birth. During a fetal resection of CCAM/CPAM, highly-skilled fetal surgeons open the mother’s abdomen and uterus in order to remove the mass from the baby’s lung. After surgery, the fetus is returned to the womb where the normal lung can continue to grow.
- EXIT procedure: If your baby has a large CCAM/CPAM that continues to grow, but hydrops does not develop, a special delivery technique called an ex utero intrapartum treatment (EXIT) procedure may be required. This delivery technique is required because their lungs are compressed and they may not be able to breathe on their own when they’re born.
In an EXIT procedure, our Garbose Family Special Delivery Unit team will partially deliver the baby so that they are still attached to the placenta and receiving oxygen through the umbilical cord. This procedure allows time for fetal surgeons to establish an airway and remove the CCAM/CPAM. After the mass is removed and your baby can breathe on their own, they will be fully delivered. - C-section to resection: More commonly, babies with large lung lesions can be safely delivered by c-section and be carried immediately to the adjacent operating room where our expert fetal surgeons will remove the mass. Our Special Delivery Unit was specifically designed with adjacent fetal operating rooms for this purpose. After the mass is removed, our dedicated Neonatal Surgical Team will provide further specialized care for your baby.
Delivery of babies with CCAM/CPAM
Most commonly, you and your medical team will make a delivery plan for 32 weeks’ gestation. Your delivery will depend on the size of the CCAM/CPAM and the course of prenatal treatment.
In the majority of cases, a mother may deliver her baby at her local hospital, without the need for high-risk neonatal care. These small lesions may no longer be visible on follow-up ultrasounds or imaging at birth, but the lesion is still present. The fetal surgeon who counseled you prenatally will determine when the lesion can be removed after birth. It is important for you to schedule a follow-up appointment once your baby is born.
Babies with larger or more complex lesions might have trouble breathing when they’re born, and should deliver in a center that offers expert care for both mother and baby in one location.
At CHOP, babies with prenatally diagnosed lung lesions who will require treatment immediately or soon after birth are delivered in our Garbose Family Special Delivery Unit, specially designed to keep mother and baby together and avoid transport of fragile infants.
Here, your baby has immediate access to our Newborn/Infant Intensive Care Unit (N/IICU) and dedicated a Neonatal Surgical Team. Our team is experienced in performing complex, delicate procedures needed to establish an airway while delivering babies who may not be able to breathe on their own at birth, as well as any immediate surgeries that your baby might need.
Surgery for CCAM/CPAM after birth

Most small lesions can be successfully treated with surgery after birth. First, you will come in for an appointment for your baby to be evaluated by the surgical team. A CT scan with contrast will be performed to confirm the diagnosis and determine the exact location of your child’s lung lesion. Children’s Hospital of Philadelphia radiologists have set the standard for safe, low-dose imaging practices in children. Surgery is typically scheduled for the same week.
At Children’s Hospital of Philadelphia, we recommend that babies with small CCAM/CPAMs undergo surgery to remove the mass when they are approximately 1 – 2 months old.
Performing the surgery when the baby is young has multiple benefits, including promoting compensatory lung growth and avoiding potential complications such as lung infections, and in rare instances, the risk of the mass turning cancerous.
The average length of stay at CHOP after lung lesion surgery is 2 – 3 days.
Follow-up care
Follow-up care for children with CCAM/CPAM will depend on the treatment the child received.
Most children treated for small lesions after birth will only need monitoring for the first year after surgery to ensure normal lung growth and lung function. The majority will require no additional long-term follow-up care.
Children treated for more severe or complex lung lesions may require ongoing monitoring through childhood. For those who experience limited lung growth resulting in pulmonary hypoplasia, CHOP’s unique Pulmonary Hypoplasia Program (PHP) offers comprehensive long-term care. The PHP team provides multidisciplinary care with a focus on improving your child’s pulmonary health, evaluating neurodevelopmental growth, meeting nutritional needs, monitoring for late onset hearing loss or any surgical issues, and more.
Long-term outlook
The vast majority of children with fetal lung lesions do extremely well and have normal lung function after their lesions are removed thanks to rapid compensatory lung growth that occurs during childhood. Having surgery early maximizes this compensatory growth.
Children with large lesions can also do extremely well, but their outlook depends on expert treatment to avoid potential complications. These babies require highly specialized expert care from the time of diagnosis to delivery and surgery to ensure the best possible long-term outcomes.
Watch the educational video below to learn more about how we diagnose and treat different fetal lung lesions at Children's Hospital of Philadelphia.

Tour our Fetal Center
The Wood Center for Fetal Diagnosis and Treatment has cared for many families and will help you through your journey, too.

What to expect
From the moment of referral through delivery and postnatal care, your family can expect a supportive experience when you come to us with a diagnosis of a birth defect.
Resources to help
CCAM/CPAM: Congenital Cystic Adenomatoid Malformation (Pulmonary Airway Malformation) Resources
Richard D. Wood Jr. Center for Fetal Diagnosis and Treatment Resources
Learning your baby has a birth defect is a life-changing experience. We want you to know that you are not alone. To help you find answers to your questions, we've created this list of educational health resources.
Reviewed by N. Scott Adzick, MD, MMM, FACS, FAAP