Individualized Medical Genetics: Athan’s Story
Published on
Published on
When Athan was an infant, his mother would carry him to the window of the Newborn/Infant Intensive Care Unit (N/IICU) at Children’s Hospital of Philadelphia (CHOP) and show him the view.
“I would tell him that one day we would take him outside where he can touch the trees,” Kristen remembers.
But the first-time mom didn’t know when, or if that would ever happen. Athan was gravely ill when he arrived at CHOP at two days old in February 2019, and doctors were searching for an answer.
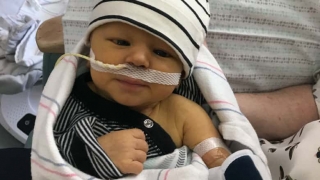 While Kristen’s pregnancy was typical, her labor wasn’t. The baby’s heart rate dropped with each contraction and when Athan was born, his skin was pale with a bluish tinge. His blood sugar tested abnormally low, even after breastfeeding, so the newborn was transferred to the central New Jersey hospital’s neonatal intensive care unit for monitoring. Late that night, a doctor woke Kristen and her husband, John. Their baby’s heel wouldn’t stop bleeding whenever they drew blood to test his sugar level. Athan would need a blood platelet transfusion.
While Kristen’s pregnancy was typical, her labor wasn’t. The baby’s heart rate dropped with each contraction and when Athan was born, his skin was pale with a bluish tinge. His blood sugar tested abnormally low, even after breastfeeding, so the newborn was transferred to the central New Jersey hospital’s neonatal intensive care unit for monitoring. Late that night, a doctor woke Kristen and her husband, John. Their baby’s heel wouldn’t stop bleeding whenever they drew blood to test his sugar level. Athan would need a blood platelet transfusion.
“It was stressful. It was not what we planned for at all,” Kristen says.
The next morning, Athan was rushed by ambulance to CHOP after an ultrasound showed a blood clot in his liver. Doctors there treated him with antibiotics, but tests quickly ruled out a virus or infection. Specialists across the hospital were brought in, but each diagnostic test result came back inconclusive.
“They reached out to every department possible. They kept testing for rarer and rarer things,” Kristen says.
Over the next two weeks, she and John watched helplessly as their son’s condition deteriorated. Athan had fevers, diarrhea, rectal bleeding, rashes and vomiting. He couldn’t tolerate food, so was fed intravenously. He needed a machine to breathe. Despite many platelet and red blood cell transfusions—36 in all—Athan’s platelet level dropped to 3 per microliter of blood, putting him at tremendous risk of bleeding. The normal range for blood platelets is 150,000-450,000.
Edward Behrens, MD, Chief of the Division of Rheumatology at CHOP was called in. Dr. Behrens heads up the Immune Dysregulation Program, which sees patients with complex immune disorders that are difficult to diagnose. It appeared that Athan had an autoimmune disorder, in which the immune system mistakenly attacks the body.
Because genetic mutations are at the root of many immune disorders, the program works closely with the Roberts Individualized Medical Genetics Center (IMGC) at CHOP. The Roberts IMGC offers genetic testing across the hospital, allowing a team of dedicated experts to explain to families how the testing might help with treatment, particularly with rare conditions, as well as facilitate state-of-the-art tests in this rapidly evolving field of study.
“If you can find the gene that’s causing the patient’s problem, there’s very likely to be a specific, hand-tailored drug that can treat that patient’s condition,” Dr. Behrens says. Otherwise, doctors are forced to rely on a “sledgehammer” approach, tamping down the symptoms with steroids and immune modulating drugs.
In Athan’s case, Dr. Behrens had an idea of what was behind the infant’s over-active immune system. He thought the Roberts IMGC could confirm his suspicion.
Five years earlier Dr. Behrens had teamed up with the Roberts IMGC to diagnose a baby girl with a mutation in the gene NLRC4 which regulates the body’s inflammatory response to foreign invaders. The gene error, which had been discovered just a year earlier, puts the immune system into overdrive, attacking the organs and causing overwhelming inflammation. The Roberts IMGC facilitated exome sequencing, for the infant, a comprehensive test that reads the genetic code of about 20,000 genes. The results showed she had the mutation.
“Finding the mutations that are meaningful is a collaborative effort between us, the diagnostic lab and the clinicians describing the symptoms,” says Livija Medne, MS, LCGC, a senior genetic counselor and co-director of the Roberts IMGC.
Hopeful, Kristen and John sat down in a conference room with Medne and Cara Skraban, MD, an attending physician and geneticist in the Roberts IMGC who recommended rapid exome sequencing, a test that returns results in roughly two weeks. The couple agreed to proceed. Blood samples were taken from Athan as well as both parents.
Nine days later, they had their results. Dr. Behrens was correct: Athan had a mutation in the NLRC4 gene. It occurred spontaneously; he did not inherit it from either of his parents.
“It was unbelievable that we finally had an answer, but we had no idea what was going to happen,” Kristen says.
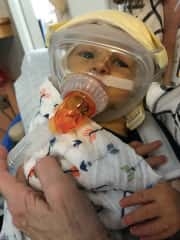 After a patient of his was diagnosed with the NLRC4 mutation in 2015, Dr. Behrens located a drug that blocks the molecules responsible for the overactive inflammatory response. He arranged for compassionate use permission to treat the baby girl with the drug. This is a special dispensation to use a medication when it has not yet been approved by the FDA. She responded so well that Dr. Behrens set up a clinical trial at CHOP.
After a patient of his was diagnosed with the NLRC4 mutation in 2015, Dr. Behrens located a drug that blocks the molecules responsible for the overactive inflammatory response. He arranged for compassionate use permission to treat the baby girl with the drug. This is a special dispensation to use a medication when it has not yet been approved by the FDA. She responded so well that Dr. Behrens set up a clinical trial at CHOP.
Athan’s parents enrolled their son in the clinical trial. Six days after his first treatment with the drug, Athan was breathing on his own. A week after that, he pulled his feeding tube out.
“We would sit there every morning with the doctors going over his numbers and everything was improving,” Kristen says. “His skin color started getting better, he was becoming more active, he started smiling. We got his first smile on camera.”
Without the Roberts IMGC on site to facilitate the rapid exome sequencing that identified the gene mutation responsible for Athan’s illness, doctors would still be searching for answers and Athan would still be very sick.
“Athan was the youngest patient ever to be enrolled in the clinical trial. And the reason why is because the Roberts IMGC allowed us to make this diagnosis so quickly,” Dr. Behrens says. “This is a profound example of how knowing the genetics makes a big difference.”
 Athan was three months old when he was strong enough to leave CHOP and go home to New Jersey. Spring was promising a new beginning and his parents couldn’t wait to show their baby boy the trees up close.
Athan was three months old when he was strong enough to leave CHOP and go home to New Jersey. Spring was promising a new beginning and his parents couldn’t wait to show their baby boy the trees up close.
“The first thing we did was put him in a stroller and take him to the park,” Kristen says.
Today Athan is a happy 1-year-old who recently took his first steps, says “momma” and “yum,” and is “100% boy,” according to his mom. Every other day, she gives him injections of the drug that put him on the road to good health—a treatment that wouldn’t have been possible without the early diagnosis available through the Roberts IMGC.
“In this case, the power of the exome sequencing was truly life altering,” Medne says. “Having an answer in nine days is really a technological breakthrough.”