Division of Urology

If your child has a urologic condition, it can be difficult to see them uncomfortable and struggling with these problems. We are here for you. As a world leader in pediatric urology, the Division of Urology at Children's Hospital of Philadelphia (CHOP) offers specialized care for a wide range of urologic disorders in children and adolescents. Our team has expertise in reconstructive surgical procedures and minimally invasive surgery. We are also skilled in the management of neurogenic bladder, kidney stones, disorders of sex development and dysfunctional voiding. We offer you and your child the ability to be seen by our dedicated pediatric urology specialists at several convenient locations throughout Pennsylvania and New Jersey.
How we serve you
Your child's urologist is an expert in diagnosing and treating the full range of urologic disorders in children and adolescents. They are also involved in developing new ways to treat these complex problems. Some of the most significant breakthroughs in treating urologic conditions have happened here.
-
Bladder Exstrophy Program -
DOVE Center for Voiding and Bladder Function -
Minimally Invasive Surgery in Pediatric Urology -
Neurogenic Bladder Program -
Occupational Therapy Services -
Pediatric Kidney Stone Center -
Urologic Oncology -
Physical Therapy Department -
Urology Psychology Services -
Urology Transitional Care Program
Conditions we treat
Our multidisciplinary team of experts specializes in diagnosing and treating the full range of urologic disorders in children and adolescents.
-
Ambiguous genitalia -
Bedwetting -
Bladder exstrophy -
Care of the uncircumcised penis -
Circumcision -
Cloacal exstrophy -
Constipation -
Daytime wetting -
Duplex kidney -
Ectopic ureter -
Epididymal cyst and spermatocele -
Epididymitis -
Epispadias -
Hematuria -
Horseshoe kidney -
Hydrocele and hernia -
Hydronephrosis/urinary tract dilation -
Hypospadias -
Kidney stones in children -
Labial adhesions -
Meatal stenosis -
Megaureter -
Micropenis -
Multicystic dysplastic kidney -
Neurogenic bladder -
Pediatric testicular tumors -
Penile adhesions -
Phimosis -
Prune belly -
PUV -
Retractile testicles -
Rhabdomyosarcoma -
Spina Bifida -
Testicular Torsion -
Torsion of the appendix testis and appendix epididymis -
Undescended testes -
Ureterocele -
Ureteropelvic junction (UPJ) obstruction -
Urethral prolapse -
Urethral stricture -
Urethrorrhagia -
Urinary frequency -
Urinary tract infection (UTI) -
Varicocele -
VUR -
Wilms tumor (kidney tumor)

Why choose us for Urology
Our program is consistently ranked one of the top pediatric urology programs in the nation. Through an integrated and compassionate approach to care, we will help your child function to their fullest potential.

Meet your team
At CHOP, your child's care is in the hands of one of the largest and most accomplished teams of pediatric urology experts in the world.

Our research
Our team of investigators conduct laboratory-based research with the goal of better understanding urologic disorders in children, remaining at the forefront of pediatric urologic care with studies such as optogenetics and advanced care for kidney disease.

Division of Urology resources
Caring for a child with an illness or injury can be overwhelming. We have resources to help you find answers to your questions and feel confident in the care you are providing your child.
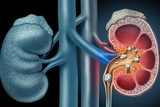
CHOP Leads Largest Study of Kidney Stone Surgeries for Children and Adolescents
Researchers at Children’s Hospital of Philadelphia (CHOP), along with several academic partners, announced the primary results of the Pediatric KIDney Stone (PKIDS) trial, the largest comparative effectiveness study of surgical interventions for children and adolescents with kidney stones.

Resources for professionals
Everything you need to support your patient’s health, created and updated by our CHOP community of experts.
News and updates
The Division of Urology at Children's Hospital of Philadelphia shares updates on research and achievements, including advancements in pediatric urology care, awards for outstanding contributions, and ongoing studies aimed at improving patient outcomes.
Your donation changes lives
A gift of any size helps us make lifesaving breakthroughs for children everywhere.


